Resumen
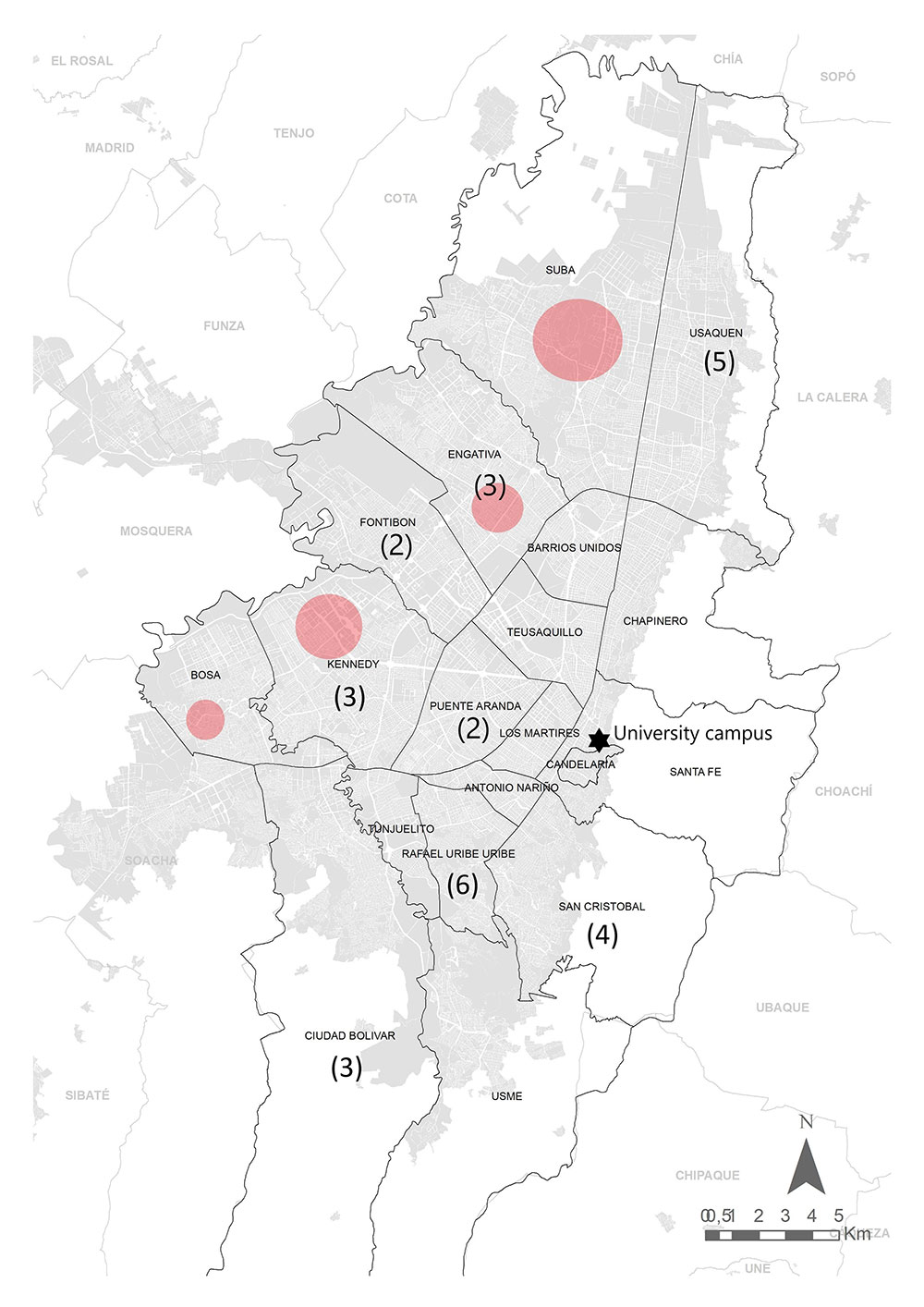
La mayoría de las evaluaciones serológicas comunitarias específicas para los anticuerpos de SARSCoV-2 se han realizado en instituciones y en trabajadores de la salud. En este estudio, se evaluaron anticuerpos IgG específicos para el virus en personas que trabajaban en una universidad de Bogotá, Colombia. El objetivo fue determinar la exposición previa a SARS-CoV-2 en los asistentes al campus durante el cierre de la ciudad. Se evaluaron 237 personas, incluidas 93 mujeres y 144 hombres, mediante la detección quimioluminiscente de anticuerpos IgG anti-proteína N viral entre noviembre y diciembre de 2020. Se encontraron 32 individuos positivos, es decir, una seroprevalencia del 13,5 % (10 mujeres y 22 hombres), en su mayoría asintomáticos (68,75 %) y se determinaron tres grupos de individuos seropositivos. Solo 13 de los individuos serorreactivos tenían una detección positiva previa del ARN del SARS-CoV-2 mediante RT-qPCR realizada, en promedio, 91 días antes de la prueba serológica. Los individuos seropositivos no provenían de las localidades con los porcentajes más altos de casos de SARS-CoV-2 en la ciudad. La encuesta se realizó después del primer pico de transmisión del SARS-CoV-2 en la ciudad y antes de la preparación para la reapertura del campus a estudiantes en el 2021, y demostró una baja seroprevalencia con un alto procentaje de asintomáticos. Estos resultados ayudarán a evaluar algunas de las estrategias establecidas para controlar la propagación del virus en instituciones educativas u otras comunidades similares.
Referencias
Ariza, B., Torres, X., Salgado, D., Cepeda, M., Gómez Restrepo, C., Castellanos, J. C., Suárez-Obando, F., Cuellar, A., Cardozo, C., Ángel, J., Franco, M. A. (2021). Seroprevalence and seroconversion rates to SARS-COV-2 in interns, residents, and medical doctors in a University Hospital in Bogotá, Colombia. Infectio. 25 (3): 145. https://doi.org/10.22354/in.v25i3.938
Bobrovitz, N., Arora, R. K., Cao, C., Boucher, E., Liu, M., Donnici, C., Yanes-Lane, M., Whelan, M., Perlman-Arrow, S., Chen, J., Rahim, H., Ilincic, N., Segal, M., Duarte, N., Wyk, J. V., Yan, T., Atmaja, A., Rocco, S., Joseph, A. Cheng, M. P. (2021). Global seroprevalence of SARS-COV-2 antibodies: A systematic review and meta-analysis. PLoS One. 16(6):e0252617. doi: 10.1371/journal.pone.0252617.
Dan, J. M., Mateus, J., Kato, Y., Hastie, K. M., Yu, E. D., Faliti, C. E., Grifoni, A., Ramirez, S.I., Haupt, S., Frazier, A., Nakao, C., Rayaprolu, V., Rawlings, S. A., Peters, B., Krammer,F., Simon, V., Saphire, E. O., Smith, D. M., Weiskopf, D., Crotty, S. (2020). Immunological memory to SARS-COV-2 assessed for up to eight months after infection. Science. 371 (6529): eabf4063. doi: 10.1126/science.abf4063.
Ebrahim, S. H., Ahmed, Q. A., Gozzer, E., Schlagenhauf, P., Memish, Z. A. (2020). Covid-19 and community mitigation strategies in a pandemic. BMJ. m1066. https://doi.org/10.1136/bmj.m1066
González, J. M., Shelton, W. J., Díaz-Vallejo, M., Rodríguez-Castellanos, V. E., Zuluaga, J. D.,Chamorro, D. F., Arroyo-Ariza, D. (2020). Analysis of commercial assays for the detection of SARS-COV-2 antibodies or antigens. Open Journal of Immunology. 10 (02): 21–35.https://doi.org/10.4236/oji.2020.102003
Guo, L., Ren, L., Yang, S., Xiao, M., Chang, D., Yang, F., Dela Cruz, C. S., Wang, Y., Wu, C., Xiao, Y., Zhang, L., Han, L., Dang, S., Xu, Y., Yang, Q.-W., Xu, S.-Y., Zhu, H.-D., Xu, Y.- C., Jin, Q., Wang, J. (2020). Profiling early humoral response to diagnose novel coronavirus disease (covid-19). Clinical Infectious Diseases. 71 (15): 778–785. https://doi.org/10.1093/cid/ciaa310
Houlihan, C. F., Vora, N., Byrne, T., Lewer, D., Kelly, G., Heaney, J., Gandhi, S., Spyer, M. J.,Beale, R., Cherepanov, P., Moore, D., Gilson, R., Gamblin, S., Kassiotis, G., McCoy, L.E., Swanton, C., Hayward, A., Nastouli, E., Aitken, J., Hatipoglu, E. (2020). Pandemic peak sars-COV-2 infection and seroconversion rates in London frontline health-care workers.The Lancet. 396 (10246). DOI: https://doi.org/10.1016/S0140-6736(20)31484-7
Instituto Nacional de Salud-INS. (2020). Covid-19 en Colombia. Coronavirus Colombia. Retrieved December 25, 2020, from https://www.ins.gov.co/Noticias/Paginas/Coronavirus.aspx.
Instituto Nacional de Salud-INS. (2021). Estudio Nacional de Seroprevalencia. Accessed on: November 12, 2021. Available from: https://www.ins.gov.co/estudio-nacional-deseroprevalencia/reporte.html
Lisboa-Bastos, M., Tavaziva, G., Abidi, S. K., Campbell, J. R., Haraoui, L.-P., Johnston, J. C., Lan, Z., Law, S., MacLean, E., Trajman, A., Menzies, D., Benedetti, A., Ahmad Khan,
F. (2020). Diagnostic accuracy of serological tests for COVID-19: Systematic Review and meta-analysis. BMJ. m2516. https://doi.org/10.1136/bmj.m2516
Laiton-Donato, K., Villabona-Arenas, C., Usme-Ciro, J., Franco-Muñoz, C., Álvarez-Díaz, D., Villabona-Arenas, L., Echeverría-Londoño, S., Cucunubá, Z., Franco-Sierra, N., Flórez, A., Ferro, C., Ajami, N., Walteros, D., Prieto, F., Durán, C., Ospina-Martínez, M., Mercado-Reyes, M. (2020). Genomic epidemiology of SARS-CoV-2 in Colombia.medRxiv. 2020.06.26.20135715 https://doi.org/10.1101/2020.06.26.20135715
Lumley, S. F., O’Donnell, D., Stoesser, N. E., Matthews, P. C., Howarth, A., Hatch, S. B., Marsden, B. D., Cox, S., James, T., Warren, F., Peck, L. J., Ritter, T. G., de Toledo, Z., Warren, L., Axten, D., Cornall, R. J., Jones, E. Y., Stuart, D. I., Screaton, G., Eyre, D. W.(2021). Antibody status and incidence of SARS-COV-2 infection in health care workers. New England Journal of Medicine. 384 (6): 533-540. https://doi.org/10.1056/nejmoa2034545
Mattar, S., Alvis-Guzmán, N., Garay, E., Rivero, R., García, A., Botero, Y., Miranda, J., Galeano, K., de La Hoz, F., Martínez, C., Arrieta, G., Faccini-Martínez, Á. A., Guzmán,
C., Kerguelen, H., Moscote, M., Contreras, H., & Contreras, V. (2020). Severe acute respiratory syndrome coronavirus 2 seroprevalence among adults in a tropical city of the Caribbean area, Colombia: Are we much closer to herd immunity than developed countries? Open Forum Infectious Diseases. 7 (12). https://doi.org/10.1093/ofid/ofaa550
Morawska, L. & Cao, J. (2020). Airborne transmission of SARS-COV-2: The world should face the reality. Environment International. 139: 105730. https://doi.org/10.1016/j.envint.2020.105730
Müller, L., Ostermann, P. N., Walker, A., Wienemann, T., Mertens, A., Adams, O., Andree, M., Hauka, S., Lübke, N., Keitel, V., Drexler, I., Di Cristanziano, V., Hermsen, D. F., Kaiser, R., Boege, F., Klein, F., Schaal, H., Timm, J., Senff, T. (2021). Sensitivity of anti-SARS-cov-2 serological assays in a high-prevalence setting. European Journal of Clinical Microbiology & Infectious Diseases. 40 (5): 1063-1071. https://doi.org/10.1007/s10096-021-04169-7
Oran, D. P. & Topol, E. J. (2021). The proportion of SARS-COV-2 infections that are asymptomatic. Annals of Internal Medicine. 174 (5): 655-662. https://doi.org/10.7326/m20-6976
Piec, I., English, E., Thomas, M. A., Dervisevic, S., Fraser, W. D., John, W. G. (2021). Performance of SARS-COV-2 serology tests: Are they good enough? PLOS ONE. 16 (2). https://doi.org/10.1371/journal.pone.0245914
Richmond, C. S., Sabin, A. P., Jobe, D. A., Lovrich, S. D., Kenny, P. A. (2020). SARS-COV-2 sequencing reveals rapid transmission from college student clusters resulting in morbidity and deaths in vulnerable populations. medRxiv 2020.10.12.20210294. https://doi.org/10.1101/2020.10.12.20210294
Roshan, R., Feroz, A. S., Rafique, Z., Virani, N. (2020). Rigorous hand hygiene practices among health care workers reduce hospital-associated infections during the COVID-19 pandemic. Journal of Primary Care & Community Health. 11: 215013272094333. https:// doi.org/10.1177/2150132720943331
Saludata. (2021). Datos en Salud Enfermedades transmisibles. Accessed on: March 28, 2021. Available from: https://saludata.saludcapital.gov.co/osb/index.php/datos-de-salud/enfermedadestrasmisibles/covid19/.
Shrock, E., Fujimura, E., Kula, T., Timms, R. T., Lee, I.-H., Leng, Y., Robinson, M. L., Sie, B.M., Li, M. Z., Chen, Y., Logue, J., Zuiani, A., McCulloch, D., Lelis, F. J., Henson, S., Monaco, D. R., Travers, M., Habibi, S., Clarke, W. A., Wong, C. (2020). Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science.370 (6520). https://doi.org/10.1126/science.abd4250
Universidad de los Andes. (2021). Universidad EN CIFRAS. Dirección de Planeación y Evaluación. Accessed on: February 8, 2021. Available from: https://planeacion.uniandes.edu.co/estadisticas/universidad-en-cifras
Walke, H. T., Honein, M. A., Redfield, R. R. (2020). Preventing and responding to COVID-19 on college campuses. JAMA. 324 (17): 1727. https://doi.org/10.1001/jama.2020.20027
Wilson, E., Donovan, C. V., Campbell, M., Chai, T., Pittman, K., Seña, A. C., Pettifor, A., Weber, D. J., Mallick, A., Cope, A., Porterfield, D. S., Pettigrew, E., Moore, Z. (2020). Multiple COVID-19 clusters on a university campus — North Carolina, August 2020. MMWR. Morbidity and Mortality Weekly Report. 69 (39): 1416–1418. https://doi.org/10.15585/mmwr.mm6939e3
World Health Organization-WHO. (2020). Diagnostic testing for SARS-COV-2. World Health Organization. Accessed on: March 25, 2021. Available from: https://www.who.int/publications-detail-redirect/diagnostic-testing-for-sars-cov-2
Xu, X., Sun, J., Nie, S., Li, H., Kong, Y., Liang, M., Hou, J., Huang, X., Li, D., Ma, T., Peng, J., Gao, S., Shao, Y., Zhu, H., Lau, J. Y.-N., Wang, G., Xie, C., Jiang, L., Huang, A., Hou, F. F. (2020). Seroprevalence of Immunoglobulin M and G antibodies against SARS-COV-2 in China. Nature Medicine. 26 (8): 1193-1195. https://doi.org/10.1038/s41591-020-0949-6
Zhao, J., Yuan, Q., Wang, H., Liu, W., Liao, X., Su, Y., Wang, X., Yuan, J., Li, T., Li, J., Qian, S., Hong, C., Wang, F., Liu, Y., Wang, Z., He, Q., Li, Z., He, B., Zhang, T., Zhang, Z. (2020). Antibody responses to SARS-COV-2 in patients with novel coronavirus disease 2019. Clinical Infectious Diseases. 71 (16): 2027-2034. https://doi.org/10.1093/cid/ciaa344
Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., Zhao, X., Huang, B., Shi, W., Lu, R., Niu, P., Zhan, F., Ma, X., Wang, D., Xu, W., Wu, G., Gao, G. F., Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine. 382 (8): 727-733. https://doi.org/10.1056/nejmoa2001017

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Derechos de autor 2021 Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales