Resumen
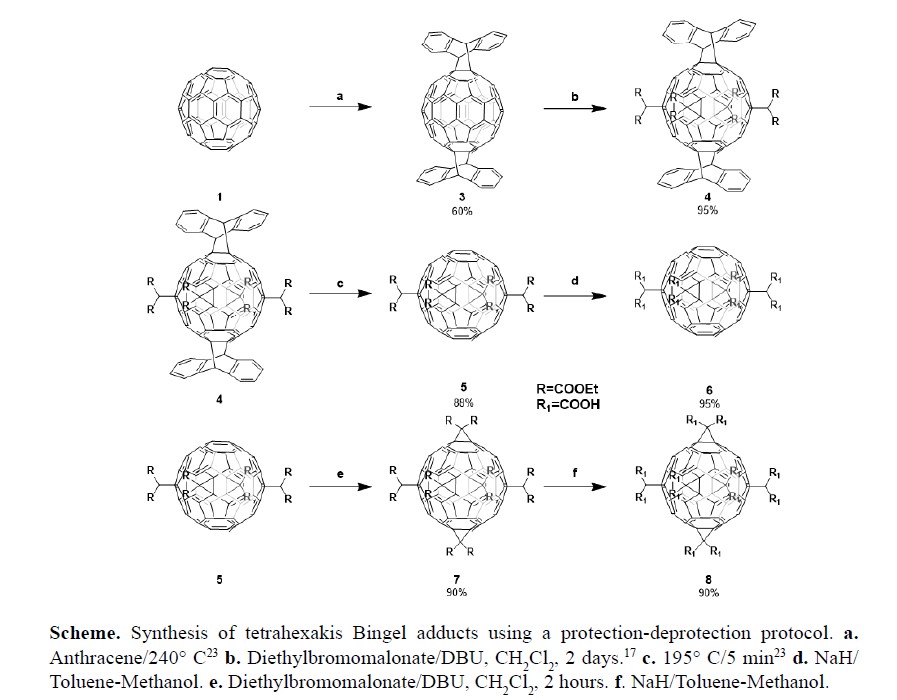
We report a new methodology for the synthesis of two highly symmetric equatorial malonate hexaadducts of C60 fullerene. The synthetic methodology is based on a series of protection and deprotection steps that allow the preparation of a fullerene [60] functionalized with six symmetrical positioned malonate addends without using complicated and expensive separation techniques (highperformance liquid chromatography, HPLC) or long reaction times. This methodology allowed us to prepare the carboxylic adducts 6 (equatorial octacarboxylic tetraadduct of C60) and 8 (equatorial dodecacarboxylic hexakisadduct of C60). As far as we now, compound 6 has not yet been reported. We also studied the electronic properties of the main compounds by UV-Vis spectroscopy and cyclic voltammetry (CV). The reported fullerene adducts exhibited several reversible reduction processes whose electron transfers are controlled by diffusion.
Referencias
Boudon, C., Gisselbrecht, J.-P., Gross, M., Isaacs, L., Anderson, H. L., Faust, R., & Diederich, F. (1995). Electrochemistry of Mono- through Hexakis-adducts of C60. Helvetica Chimica Acta. 78 (5): 1334-1344. Doi: 10.1002/hlca.19950780523
Buntar, V. & Weber, H. W. (1996). Magnetic properties of fullerene superconductors. Superconductor Science and Technology. 9 (8): 599-615. Doi: 10.1088/0953-2048/9/8/001
Cardullo, F, Seiler, P., Isaacs, L., Nierengarten, J. F., Haldimann, R. F., Diederich, F., MordasiniDenti, T., Thiel, W., Boudon, C., Gisselbrecht, J. P., Gross, M. (1997). Bisthrough tetrakis-adducts of C-60 by reversible tether-directed remote functionalization and systematic investigation of the changes in fullerene properties as a function of degree, pattern, and nature of functionalization. Helvetica Chimica Acta. 80 (2): 343-371. Doi: 10.1002/hlca.19970800203
Cardullo, Francesca, Isaacs, L., Diederich, F., Gisselbrecht, J.-P., Boudon, C., Gross, M. (1996). Regiospecific templated synthesis of D 2h-symmetrical tetrakis-adduct C64(COOEt)8 by reversible tether-directed remote functionalization of C60. Chemical Communications. 12(6): 797. Doi: 10.1039/cc9960000797
Castro, E., García, A. H., Zavala, G., Echegoyen, L. (2017). Fullerenes in biology and medicine. Journal of Materials Chemistry B. 5 (32): 6523-6535. Doi: 10.1039/c7tb00855d
Castro, E., Murillo, J., Fernandez-Delgado, O., Echegoyen, L. (2018). Progress in fullerenebased hybrid perovskite solar cells. Journal of Materials Chemistry C. 6 (11): 2635-2651. Doi: 10.1039/c7tc04302c
Chen, C.-H., Aghabali, A., Metta-Magana, A. J., Olmstead, M. M., Balch, A. L., Echegoyen, L. (2015). Synthesis and characterization of a trans-1 hexakis-fullerene linker that forms crystalline polymers with silver salts. Dalton Transactions. 44 (42): 18487-18491. Doi:10.1039/C5DT03054D
Collavini, S. & Delgado, J. L. (2018). Fullerenes: The stars of photovoltaics. Sustainable Energy and Fuels. 2 (11): 2480-2493. Doi: 10.1039/c8se00254a
Djojo, F., Herzog, A., Lamparth, I., Hampel, F., Hirsch, A. (1996). Regiochemistry of twofold additions to [6,6] bonds in C60: Influence of the addend-independent cage distortion in 1,2-monoadducts. Chemistry- A European Journal. 2 (12): 1537- 547. Doi: 10.1002/chem.19960021211
Duarte-Ruiz, Á., Echegoyen, L., Aya, A., Gómez-Baquero, F. (2009). A New Method to prepare an e , e , e Trisadduct of C 60 Using a Protection- Deprotection Sequence. J. Mex. Chem. Soc. 53 (3): 169-173.
Duarte-Ruiz, A., Wurst, K., Kräutler, B. (2001). Regioselective “one-pot” synthesis of antipodal bis-adducts by heating of solid [5,6]fullerene-C60-Ih and anthracenes. Helvetica Chimica Acta. 84 (8): 2167-2177. Doi: 10.1002/1522-2675(20010815)84:8<2167::AID-HLCA2167>3.0.CO;2-V
Duarte-Ruiz, A., Wurst, K., Kräutler, B. (2008). The Orthogonal (e, e, e)-Tris-Adduct of 9,10-Dimethylanthracene with C 60 -Fullerene: A Hidden Cornerstone of Fullerene Chemistry. Preliminary Communication. Helvetica Chimica Acta. 91 (8): 1401- 408. Doi: 10.1002/hlca.200890152
Dugan, L. L., Turetsky, D. M., Du, C., Lobner, D., Wheeler, M., Almli, C. R., Shen, C. K.-F., Luh, T.-Y., Choi, D. W., Lin, T.-S. (1997). Carboxyfullerenes as neuroprotective agents. Proceedings of the National Academy of Sciences. 94 (17): 9434-9439. Doi: 10.1073/pnas.94.17.9434
Elsevier B.V. (2019). Scopus - Analyze search results key word: fullerene. Analyze Search Results. Key Word: Fullerene.https://usc.elogim.com:2092/term/analyzer.uri?id=f85731bec23ed1fe941672a4746a9b3b&origin=resultslist&src=s&s=TITLE-ABS-KEY%28fullerene%29&sort=plf-&sdt=b&sot=b&sl=24&count=40245&analyzeResults=Analyze+results&txGid=77bc34c7a17bf5c7e8abdedbc0902929Accesed2019-03-25
Giacalone, F. & Martín, N. (2006). Fullerene polymers: Synthesis and properties. In Chemical Reviews, American Chemical Society. 106 (12): 5136-5190. Doi: 10.1021/cr068389h
Hirsch, A., Lamparth, I., Grösser, T., Karfunkel, H. R. (1994). Regiochemistry of Multiple Additions to the Fullerene Core: Synthesis of a Tb-Symmetric Hexakisadduct of C60with Bis(ethoxycarbonyl)methylene. Journal of the American Chemical Society. 116 (20): 9385-9386. Doi: 10.1021/ja00099a088
Hirsch, A., Lamparth, I., Karfunkel, H. R. (1994). Fullerene Chemistry in Three Dimensions: Isolation of Seven Regioisomeric Bisadducts and Chiral Trisadducts of C60 and Di(ethoxycarbonyl)methylene. Angewandte Chemie International Edition in English. 33 (4): 437-438. Doi: 10.1002/anie.199404371
Hirsch, A. & Vostrowsky, O. (2001). C60 Hexakisadducts with an Octahedral Addition Pattern −A New Structure Motif in Organic Chemistry. European Journal of Organic Chemistry. 2001 (5): 829-848. Doi: 10.1002/1099-0690(200103)2001:5<829::AID-EJOC829>3.0.CO;2-V
Kop, T., Bjelaković, M., Milić, D. (2015). Synthesis and properties of bis(pyrrolidino)fullerenes bridged by a flexible alkyl-tether. Tetrahedron. 71 (29): 4801-4809. Doi: 10.1016/j.tet.2015.05.038
Kraft, A. & Beuerle, F. (2016). Metal–organic hybrid architectures built from functionalized fullerenes and metal ions or clusters. Tetrahedron Letters. 57 (42): 4651-4663. Doi: 10.1016/J.TETLET.2016.08.082
Kraft, A., Gsänger, M., Beuerle, F. (2014). Arranging Fullerenes through Hydrogen Bonding. European Journal of Organic Chemistry. 2014 (3): 523-528. Doi: 10.1002/ejoc.201301298
Kraft, A., Stangl, J., Krause, A., Müller-buschbaum, K., Beuerle, F. (2017). Supramolecular frameworks based on [ 60 ] fullerene hexakisadducts. Beilstein J. Org. Chem. 13 (1): 1-9. Doi: 10.3762/bjoc.13.1
Krätschmer, W., Lamb, L. D., Fostiropoulos, K., Huffman, D. R. (1990). Solid C60: a new form of carbon. Nature. 347 (6291): 354-358. Doi: 10.1038/347354a0
Kräutler, B., Müller, T., Duarte-Ruiz, A. (2001). Efficient preparation of monoadducts of [60] fullerene and anthracenes by solution chemistry and their thermolytic decomposition in the solid state. Chemistry - A European Journal. 7 (15): 3223-235. Doi: 10.1002/1521-3765 (20010803)7:15<3223::AID-CHEM3223>3.0.CO;2-B
Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F., Smalley, R. E. (1985). C60: Buckminsterfullerene. Nature. 318 (6042): 162–163. Doi: 10.1038/318162a0
Lamparth, I. & Hirsch, A. (1994). Water-soluble Malonic Acid Derivatives of. J. Chem. Soc., Chem. Commun. 7: 1727-1728.
Lamparth, I., Maichle–Mössmer, C., Hirsch, A. (1995). Reversible Template-Directed Activation of Equatorial Double Bonds of the Fullerene Framework: Regioselective Direct Synthesis, Crystal Structure, and Aromatic Properties ofTh-66(COOEt)12. Angewandte Chemie International Edition in English. 34 (15): 1607-1609. Doi: 10.1002/anie.199516071
Li, H., Haque, S. A., Kitaygorodskiy, A., Meziani, M. J., Torres-Castillo, M., Sun, Y.-P. (2006). Alternatively Modified Bingel Reaction for Efficient Syntheses of C 60 Hexakis- Adducts. Organic Letters. 8 (24): 5641-5643. Doi: 10.1021/ol062391d
Lu, Q., Schuster, D. I., Wilson, S. R. (1996). Preparation and Characterization of Six Bis( N-methylpyrrolidine)−C 60 Isomers: Magnetic Deshielding in Isomeric Bisadducts of C 60. The Journal of Organic Chemistry. 61 (14): 4764-4768. Doi: 10.1021/jo960466t
Ma, N., Lv, M., Liu, T., Song, M., Liu, Y., Zhang, G. (2019). Second-order nonlinear optical properties of [60]fullerene-fused dihydrocarboline derivates: A theoretical study on switch effect. Journal of Materials Chemistry C. 7 (42): 13052-13058. Doi: 10.1039/c9tc04126e
Martínez, Z. S., Castro, E., Seong, C.-S., Cerón, M. R., Echegoyen, L., Llano, M. (2016). Fullerene Derivatives Strongly Inhibit HIV-1 Replication by Affecting Virus Maturation without Impairing Protease Activity. Antimicrobial Agents and Chemotherapy. 60 (10):5731-5741. Doi: 10.1128/AAC.00341-16
Minar, N. K., Hou, K., Westermeier, C., Döblinger, M., Schuster, J., Hanusch, F. C., Nickel, B., Ozin, G. A., Bein, T. (2015). A Highly-Ordered 3D Covalent Fullerene Framework. Angewandte Chemie. 127 (26): 7687-7691. Doi: 10.1002/ange.201411344
Nierengarten, J. F., Armaroli, N., Accorsi, G., Rio, Y., Eckert, J. F. (2003). [60] Fullerene: A versatile photoactive core for dendrimer chemistry. Chemistry - A European Journal. 9 (1):36-41. Doi: 10.1002/chem.200390001
Ohsawa, Y. & Saji, T. (1992). Electrochemical detection of C60^6- at low temperature. Journal of the Chemical Society, Chemical Communications. 0 (10): 781. Doi: 10.1039/c39920000781
Ortiz, A. L., Rivera, D. M., Athans, A. J., Echegoyen, L. (2009). Regioselective addition of N-(4-Thiocyanatophenyl)pyrrolidine addends to fullerenes. European Journal of Organic Chemistry. 20: 3396-3403. Doi: 10.1002/ejoc.200900228
Paolucci, F., Carano, M., Ceroni, P., Mottier, L., Sergio, R. (1999). Electrochemical Detection of C[sub 60] in Solution: Is Tetrahydrofuran a Suitable Solvent for Fullerene Studies? Journal of The Electrochemical Society. 146 (9): 3357-3360. Doi: 10.1149/1.1392477
Peng, P., Li, F.-F., Neti, V. S. P. K., Metta-Magana, A. J., Echegoyen, L. (2014). Design, Synthesis, and X-Ray Crystal Structure of a Fullerene-Linked Metal-Organic Framework. Angewandte Chemie International Edition. 53 (1): 160-163. Doi: 10.1002/anie.201306761
Peng, P., Li, F. F., Bowles, F. L., Neti, V. S. P. K., J. Metta-Magana, A., Olmstead, M. M., Balch, A. L., Echegoyen, L. (2013). High yield synthesis of a new fullerene linker and its use in the formation of a linear coordination polymer by silver complexation. Chemical Communications. 49 (31): 3209-3211. Doi: 10.1039/c3cc40697k
Richardson, C. F., Schuster, D. I., Wilson, S. R. (2000). Synthesis and characterization of water-soluble amino fullerene derivatives. Organic Letters. 2 (8): 1011-1014. Doi: 10.1021/ol990312a
Romero, E. L., Cabrera-Espinoza, A., Ortiz-Peña, N., Soto-Monsalve, M., Zuluaga, F., D’Vries, R. F., Chaur, M. N. (2017a). New pyrazolino and pyrrolidino[60]fullerenes: the introduction of the hydrazone moiety for the formation of metal complexes. Journal of Physical Organic Chemistry. 30 (2): e3601. Doi: 10.1002/poc.3601
Romero, E. L., Cabrera-Espinoza, A., Ortiz-Peña, N., Soto-Monsalve, M., Zuluaga, F., D’Vries, R. F., Chaur, M. N. (2017b). New pyrazolino and pyrrolidino[60]fullerenes: the introduction of the hydrazone moiety for the formation of metal complexes. Journal of Physical Organic Chemistry. 30 (2): 3601-3608. Doi: 10.1002/poc.3601
Sandoval, J., Ventura-Sobrevilla, J., Boone-Villa, D., Ramos-González, R., Velázquez, M., SilvaBelmares, Y., Cobos-Puc, L., Aguilar, C. (2019). Carbon nanomaterials as pharmaceutic forms for sustained and controlled delivery systems. In Nanomaterials for Drug Delivery and Therapy. Chapter 14. Editor: Alexandru Mihai Grumezescu. William Andrew Publishing, p. 403-434. ISBN 9780128165058. Doi: 10.1016/b978-0-12-816505-8.00003-5
Schwenninger, R., Muller, T., Krautler, B. (1997). Concise route to symmetric multiadducts of [60]fullerene: Preparation of an equatorial tetraadduct by orthogonal transposition. Journal of the American Chemical Society. 119 (39): 9317-9318. Doi: 10.1021/ja971875p
Teprovich, J. A., Weeks, J. A., Ward, P. A., Tinkey, S. C., Huang, C., Zhou, J., Zidan, R., Jena, P. (2019). Hydrogenated C60 as High-Capacity Stable Anode Materials for Li Ion Batteries [Research-article]. ACS Applied Energy Materials. 2 (9): 6453- 460. Doi: 10.1021/acsaem.9b01040
Ugan, L. A. L. D., Uretsky, D. O. M. T., Heng, C. D. U., Obner, D. O. U. G. L., Heeler, M. A. R. K. W. (1997). Carboxyfullerenes as neuroprotective agents. Proceedings of the National Academy of Sciences of the United States of America. 94 (August): 9434-9439. Doi: 10.1073/pnas.94.17.9434
Wang, L., Ye, J.-T., Wang, H.-Q., Xie, H.-M., Qiu, Y.-Q. (2018). Third-Order Nonlinear Optical Properties of Endohedral Fullerene (H 2 ) 2 @C 70 and (H 2 O) 2 @C 70 Accompanied by the Prospective of Novel (HF) 2 @C 70. The Journal of Physical Chemistry C. 122 (12):6835-6845. Doi: 10.1021/acs.jpcc.8b00623
Xie, Q., Pérez-Cordero, E., Echegoyen, L. (1992). Electrochemical Detection of C606-and C706-: Enhanced Stability of Fullerides in Solution. Journal of the American Chemical Society. 114 (10): 3978-3980. Doi: 10.1021/ja00036a056
Ya. Vul, A. (2002). Perspectives of Fullerene Nanotechnology. In E. Ōsawa (Ed.), Perspectives of Fullerene Nanotechnology. Springer Netherlands. Doi: 10.1007/978-94-010-9598-3
Zhang, S., Lukoyanova, O., Echegoyen, L. (2006). Synthesis of fullerene adducts with terpyridyl-or pyridylpyrrolidine groups in trans-1 positions. Chemistry - A European Journal. 12 (10):2846-2853. Doi: 10.1002/chem.200501333
Zhou, Z., Sarova, G. H., Zhang, S., Ou, Z., Tat, F. T., Kadish, K. M., Echegoyen, L., Guldi, D. M., Schuster, D. I., Wilson, S. R. (2006). Fullerene polypyridine ligands: Synthesis, ruthenium complexes, and electrochemical and photophysical properties. Chemistry - A European Journal. 12 (16): 4241-4248. Doi: 10.1002/chem.200600021

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
Derechos de autor 2021 Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales