Resumen
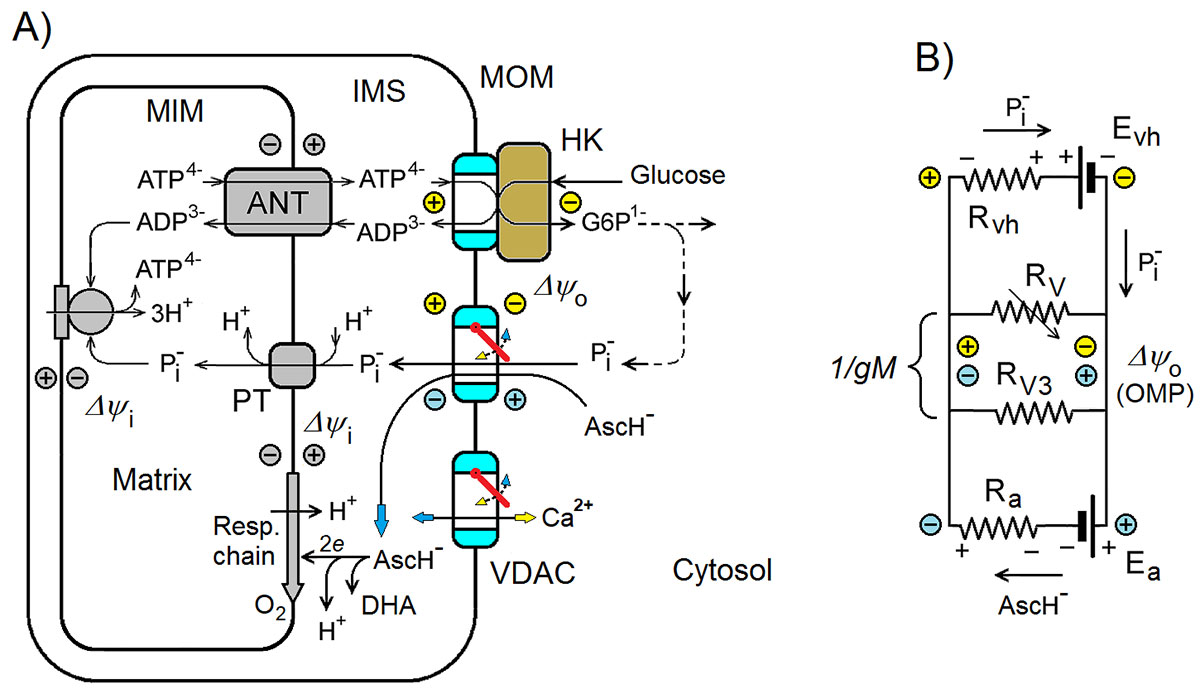
La característica principal de los tumores malignos de crecimiento rápido es el metabolismo de tipo Warburg, directamente relacionado con una cantidad extremadamente alta de hexoquinasa (HQ) unida a los canales aniónicos dependientes de voltaje (VDAC) en la membrana mitocondrial externa. En un estudio anterior se explicó el metabolismo de tipo Warburg como resultado de la supresión eléctrica de las mitocondrias debido al cierre de los VDAC libres, no unidos a HQ. En este se propone un posible nuevo mecanismo de la actividad anticancerígena de altas dosis de vitamina C (ascorbato) estimado mediante un modelo computacional simplificado. Según la hipótesis propuesta y el modelo, la oxidación de ascorbato en las mitocondrias conduce a la generación del potencial negativo de la membrana externa (PME), de signo opuesto al PME positivo generado por los complejos VDAC-HQ en las células cancerosas. El modelo demuestra que el PME negativo, incluso de magnitudes relativamente bajas, generado mediante cualquier mecanismo, lleva a la reapertura de los VDAC cerrados eléctricamente, reprogramando así el metabolismo energético celular. Según la hipótesis, los mediadores redox, que aumentan la tasa de oxidación de ascorbato en las mitocondrias, deberían aumentar sinérgicamente los efectos anticancerígenos de las dosis altas de ascorbato en concordancia con los datos experimentales reportados en la literatura. El modelo muestra que, incluso pequeños cambios en la sensibilidad al voltaje de los VDAC y/o de la cantidad de los complejos VDAC-HQ, causados por diversos factores fisiológicos como se sabe, podrían influir fuertemente en el mecanismo mitocondrial propuesto de la actividad anticancerígena del ascorbato.
Referencias
Abramczyk, H., Surmacki, J.M., Brozek-Pluska, B., Kopec, M. (2021). Revision of commonly accepted Warburg mechanism of cancer development: redox-sensitive mitochondrial cytochromes in breast and brain cancers by Raman imaging. Cancers (Basel). 13 (11): 2599. Doi:10.3390/cancers13112599
Bakalova, R., Zhelev, Z., Miller, T., Aoki, I., Higashi, T. (2020). Vitamin C versus cancer: Ascorbic acid radical and impairment of mitochondrial respiration? Oxid. Med. Cell. Longev. 2020:1504048. Doi: 10.1155/2020/1504048
Bazzan, A.J., Zabrecky, G., Wintering, N., Newberg, A.B., Monti, D.A. (2018). Retrospective evaluation of clinical experience with intravenous ascorbic acid in patients with cancer.Integr. Cancer Ther. 17 (3): 912-920. Doi: 10.1177/1534735418775809
Bernardi, P. (2013). The mitochondrial permeability transition pore: a mystery solved? Front.Physiol. 4: 95. Doi: 10.3389/fphys.2013.00095
Blaszczak, W., Barczak, W., Masternak, J., Kopczyński, P., Zhitkovich, A., Rubiś, B. (2019).Vitamin C as a modulator of the response to cancer therapy. Molecules. 24 (3): 453. Doi:10.3390/molecules24030453
Camara, A.K.S., Zhou, Y., Wen, P.C., Tajkhorshid, E., Kwok, W.M. (2017). Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front. Physiol. 8: 460. Doi: 10.3389/fphys.2017.00460
Cameron, E. & Pauling, L. (1976). Supplemental ascorbate in the supportive treatment of cancer: prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U.S.A. 73:3685–3689. Doi: 10.1073/pnas.73.10.3685
Cameron, E. & Pauling, L. (1978). Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. U.S.A. 75: 4538-4542. Doi: 10.1073/pnas.75.9.4538
Carosio, R., Zuccari, G., Orienti, I., Mangraviti, S., Montaldo, P.G. (2007). Sodium ascorbate induces apoptosis in neuroblastoma cell lines by interfering with iron uptake. Mol. Cancer. 6:55. Doi: 10.1186/1476-4598-6-55
Chen, Q., Espey, M. G., Sun, A. Y., Pooput, C., Kirk, K. L., Krishna, M. C., Khosh, D. B., Drisko J., Levine, M. (2008). Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. U.S.A. 105: 11105-11109. Doi: 10.1073/pnas.0804226105
Ciscato, F., Filadi, R., Masgras, I., Pizzi, M., Marin, O., Damiano, N., Pizzo, P., Gori, A., Frezzato, F., Chiara, F., Trentin, L., Bernardi, P., Rasola A. (2020). Hexokinase 2 displacement from mitochondria-associated membranes prompts Ca(2+)-dependent death of cancer cells. EMBO Rep. 21 (7): e49117, Doi: 10.15252/embr.201949117
Colombini, M. (2016). The VDAC channel: Molecular basis for selectivity. Biochim. Biophys. Acta. 1863 (10): 2498-2502. Doi: 10.1016/j.bbamcr.2016.01.019
De Pinto, V., Reina, S., Gupta, A., Messina, A., Mahalakshmi, R. (2016). Role of cysteines in mammalian VDAC isoforms’ function. Biochim. Biophys. Acta. 1857 (8): 1219-1227. Doi:10.1016/j.bbabio.2016.02.020
Feichtinger, R.G., Weis, S., Mayr, J.A., Zimmermann, F., Geilberger, R., Sperl, W., Kofler, B.(2014). Alterations of oxidative phosphorylation complexes in astrocytomas. Glia. 62: 514-525. Doi. 10.1002/glia.22621
Fulda, S. (2009). Tumor resistance to apoptosis. Int. J. Cancer. 124: 511-515. Doi: 10.1002/ijc.24064
Galluzzi, L., Kepp, O., Tajeddine, N., Kroemer, G. (2008). Disruption of the hexokinase-VDAC complex for tumor therapy. Oncogene. 27: 4633-4635. Doi: 10.1038/onc.2008.114
Giansanti, M., Karimi, T., Faraoni, I., Graziani, G. (2021). High-dose vitamin C: Preclinical evidence for tailoring treatment in cancer patients. Cancers (Basel). 13 (6): 1428. Doi:10.3390/cancers13061428
Gilloteaux, J., Jamison, J.M., Arnold, D., Neal, D.R., Summers, J.L. (2006). Morphology and DNA degeneration during autoschizic cell death in bladder carcinoma T24 cells induced by ascorbate and menadione treatment. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288 (1): 58-83. Doi: 10.1002/ar.a.20276
Gogvadze, V., Orrenius, S., Zhivotovsky, B. (2008). Mitochondria in cancer cells: what is so special about them? Trends in Cell Biol. 18 (4): 165-173. Doi: 10.1016/j.tcb.2008.01.006
González, M.J., Rosario-Pérez, G., Guzmán, A.M., Miranda-Massari, J.R., Duconge, J., Lavergne, J., Fernandez, N., Ortiz, N., Quintero, A., Mikirova, N., Riordan, N.H., Ricart, C.M. (2010). Mitochondria, energy and cancer: The relationship with ascorbic acid. J. Orthomol. Med. 25 (1): 29-38.
Gonzalez, M.J., Miranda Massari, J.R., Duconge, J., Riordan, N.H., Ichim, T., Quintero-Del-Rio, A.I., Ortiz, N. (2012). The bio-energetic theory of carcinogenesis. Med. Hypotheses. 79 (4): 433-439. Doi: 10.1016/j.mehy.2012.06.015
Grimm, S. & Brdiczka, D. (2007). The permeability transition pore in cell death. Apoptosis. 12 (5):841-855. Doi: 10.1007/s10495-007-0747-3
Han, D., Antunes, F., Canali, R., Rettori, D., Cadenas, E. (2003). Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 278 (8): 5557-5563. Doi: 10.1074/jbc.M210269200
Hill, B.C. & Nicholls, P. (1980). Reduction and activity of cytochrome c in the cytochrome ccytochrome aa3 complex. Biochem. J. 187 (3): 809-818. Doi: 10.1042/bj1870809
Hua, A.B., Justiniano, R., Perer, J., Park, S.L., Li, H., Cabello, C.M., Wondrak, G.T. (2019).Repurposing the electron transfer reactant phenazine methosulfate (PMS) for the apoptotic elimination of malignant melanoma cells through induction of lethal oxidative and mitochondriotoxic stress. Cancers (Basel). 11 (5): 590. Doi: 10.3390/cancers11050590
Kang, J.S., Cho, D., Kim, Y.I., Hahm, E., Yang, Y., Kim, D., Hur, D., Park, H., Bang, S., Hwang,Y.I., Lee, W.J. (2003). L-ascorbic acid (vitamin C) induces the apoptosis of B16 murine melanoma cells via a caspase-8-independent pathway. Cancer Immunol. Immunother. 52 (11): 693-698. Doi: 10.1007/s00262-003-0407-6
Lee, A.C., Xu, X., Colombini, M. (1996). The role of pyridine dinucleotides in regulating the permeability of the mitochondrial outer membrane. J. Biol. Chem. 271 (43): 26724-26731.Doi: 10.1074/jbc.271.43.26724
Lehninger, A.L., UL Hassan, M., Sudduth, H.C. (1954). Phosphorylation coupled to the oxidation of ascorbate by isolated mitochondria. J. Biol. Chem. 210 (2): 911-922. Doi: 10.1016/S0021-9258(18)65418-3
Lemeshko, V.V. (2002). Model of the outer membrane potential generation by the inner membrane of mitochondria. Biophys. J. 82: 684-692. Doi: 10.1016/S0006-3495(02)75431-3
Lemeshko, V.V., Haridas, V., Quijano-Pérez, J.C., Gutterman, J.U. (2006). Avicins, natural anticancer saponins, permeabilize mitochondrial membranes. Arch. Biochem. Biophys. 454:114-122. Doi: 10.1016/j.abb.2006.08.008
Lemeshko, V.V. (2014). VDAC electronics: 1. VDAC-hexo(gluco)kinase generator of the mitochondrial outer membrane potential. Biochim. Biophys. Acta. 1838: 1362-1371. Doi:10.1016/j.bbamem.2014.01.001
Lemeshko, V. (2015). The Warburg effect as a VDAC-hexokinase-mediated electrical suppression of mitochondrial energy metabolism. FASEB J. 29 (Suppl. 1): 725.27.
Lemeshko, V.V. (2017). The mitochondrial outer membrane potential as an electrical feedback control of cell energy metabolism. In: T.K. Rostovtseva (Ed.), Molecular Basis for Mitochondrial Signaling. Springer International Publishing, New York, pp. 217-250. Chapter 9.
Lemeshko, V. (2018). The role of the mitochondrial outer membrane in the control of cell energy metabolism. Rev. Acad. Colomb. Cienc. Exact. Fis. Nat. 42 (162): 6-21 (Spanish). Doi:10.18257/raccefyn.549
Lemeshko, V.V. (2021). Electrical control of the cell energy metabolism at the level of mitochondrial outer membrane. BBA – Biomembranes. 1863: 183493. Doi: 10.1016/j.bbamem.2020.183493
Lv, H., Wang, C., Fang, T., Li, T., Lv, G., Han, Q., Yang, W., Wang, H. (2018). Vitamin C preferentially kills cancer stem cells in hepatocellular carcinoma via SVCT-2. NPJ Precis.Oncol. 2 (1): 1. Doi: 10.1038/s41698-017-0044-8
Ma, E., Chen, P., Wilkins, H.M., Wang, T., Swerdlow, R.H., Chen, Q. (2017). Pharmacologic ascorbate induces neuroblastoma cell death by hydrogen peroxide mediated DNA damage and reduction in cancer cell glycolysis. Free Radic. Biol. Med. 113: 36-47. Doi: 10.1016/j.freeradbiomed.2017.09.008
Magrì, A., Reina, S., De Pinto, V. (2018). VDAC1 as pharmacological target in cancer and neurodegeneration: focus on its role in apoptosis. Front. Chem. 6: 108. Doi: 10.3389/fchem.2018.00108
Maldonado, E.N., Sheldon, K.L., DeHart, D.N., Patnaik, J., Manevich, Y., Townsend, D. M.,Bezrukov, S.M., Rostovtseva, T.K., Lemasters, J.J. (2013). Voltage-dependent anion channels modulate mitochondria metabolism in cancer cells: regulation by free tubulin and erastin. J. Biol. Chem. 288 (17): 11920-11929. Doi: 10.1074/jbc.M112.433847
Marín-Hernández, A., Rodríguez-Enríquez, S., Vital-González, P.A., Flores-Rodríguez, F.L., Macías-Silva, M., Sosa-Garrocho, M., Moreno-Sánchez, R. (2006). Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J. 273: 1975-1988. Doi:10.1111/j.1742-4658.2006.05214.x
McCormick, W.J. (1954). Cancer: the preconditioning factor in pathogenesis; a new etiologic approach. Arch. Pediatr. 71: 313-322.
McCormick, W.J. (1959). Cancer: a collagen disease, secondary to a nutritional deficiency. Arch.Pediatr. 76 (4): 166-171.
Nakashima, R.A., Paggi, M.G., Scott, L.J., Pedersen, P.L. (1988). Purification and characterization of a bindable form of mitochondrial bound hexokinase from the highly glycolytic AS-30D rat hepatoma cell line. Cancer Res. 48: 913-919.
Ngo, B., van Riper, J., Cantley, L.C., Yun, J. (2019). Targeting cancer vulnerabilities with highdose vitamin C. Nature Reviews Cancer. 19 (5): 271-282. Doi: 10.1038/s41568-019-0135-7
Nicholls, P., Hildebrandt, V., Hill, B.C., Nicholls, F., Wrigglesworth, J.M. (1980). Pathways of cytochrome c oxidation by soluble and membrane-bound cytochrome aa3. Can. J. Biochem.58: 969-977. Doi: 10.1139/o80-132
Noto, V., Taper, H.S., Jiang, Y.H., Janssens, J., Bonte, J., De Loecker, W. (1989). Effects of sodium ascorbate (vitamin C) and 2-methyl-1,4-naphthoquinone (vitamin K3) treatment on human tumor cell growth in vitro. I. Synergism of combined vitamin C and K3 action. Cancer. 63 (5): 901-906. Doi: 10.1002/1097-0142(19890301)63:5<901::aid-cncr2820630518>3.0.co;2-g
Park, S. (2013). The effects of high concentrations of vitamin C on cancer cells. Nutrients. 5 (9):3496-3505. Doi: 10.3390/nu5093496
Polireddy, K., Dong, R., Reed, G., Yu, J., Chen, P., Williamson, S., Violet, P.C., Pessetto, Z., Godwin, A.K., Fan, F., Levine, M., Drisko, J.A., Chen, Q. (2017). High dose parenteral ascorbate inhibited pancreatic cancer growth and metastasis: Mechanisms and a phase I/IIa study. Sci. Rep. 7 (1): 17188. Doi: 10.1038/s41598-017-17568-8
Roa, F.J., Peña, E., Gatica, M., Escobar-Acuña, K., Saavedra, P., Maldonado, M., Cuevas, M.E., Moraga-Cid, G., Rivas, C.I., Muñoz-Montesino, C. (2020). Therapeutic use of vitamin C in cancer: Physiological considerations. Front. Pharmacol. 11: 211. Doi: 10.3389/fphar.2020.00211
Rostovtseva, T. & Colombini, M. (1997). VDAC channels mediate and gate the flow of ATP:implications for the regulation of mitochondrial function. Biophys. J. 72 (5): 1954-1962. Doi: 10.1016/S0006-3495(97)78841-6
Rostovtseva, T.K., Queralt-Martín, M., Rosencrans, W.M., Bezrukov, S.M. (2020). Targeting the multiple physiologic roles of VDAC with steroids and hydrophobic drugs. Front. Physiol. 11:446. Doi: 10.3389/fphys.2020.00446
Rostovtseva, T.K., Bezrukov, S.M., Hoogerheide, D.P. (2021). Regulation of Mitochondrial Respiration by VDAC Is Enhanced by Membrane-Bound Inhibitors with Disordered Polyanionic C-Terminal Domains. Int. J. Mol. Sci. 22 (14): 7358. Doi: 10.3390/ijms22147358
Sanadi, D.R. (1964). On the mechanism of oxidative phosphorylation IX. Energy-dependent reduction of nicotinamide adenine dinucleotide by ascorbate and ubiquinone. Biochim.Biophys. Acta. 89: 367-369. Doi: 10.1016/0926-6569(64)90231-7
Semkova, S., Zhelev, Z., Miller, T., Sugaya, K., Aoki, I., Higashi, T., Bakalova, R. (2020).Menadione/Ascorbate induces overproduction of mitochondrial superoxide and impairs mitochondrial function in cancer: comparative study on cancer and normal cells of the same origin. Anticancer Res. 40 (4): 1963-1972. Doi: 10.21873/anticanres.14151
Shteinfer-Kuzmine, A., Amsalem, Z., Arif, T., Zooravlov, A., Shoshan-Barmatz, V. (2018).Selective induction of cancer cell death by VDAC1-based peptides and their potential use in cancer therapy. Mol. Oncol. 12 (7): 1077-1103. Doi: 10.1002/1878-0261.12313
Sun, L., Shukair, S., Naik, T.J., Moazed, F., Ardehali, H. (2008). Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol.Cell. Biol. 28 (3): 1007-1017. Doi: 10.1128/MCB.00224-07
Tan, W. & Colombini, M. (2007). VDAC closure increases calcium ion flux. Biochim. Biophys.Acta. 1768 (10): 2510-2515. Doi: 10.1016/j.bbamem.2007.06.002
Thayer, W.S. & Rubin, E. (1981). Molecular alterations in the respiratory chain of rat liver after chronic ethanol consumption. J. Biol. Chem. 256 (12): 6090-6097.
Tomasetti, M., Santarelli, L., Alleva, R., Dong, L.-F., Neuzil, J. (2015). Redox-active and redoxsilent compounds: synergistic therapeutics in cancer. Curr. Med. Chem. 22 (5): 552-568. Doi:10.2174/0929867321666140915142219
Valenti, D., de Bari, L., De Filippis, B., Ricceri, L., Vacca, R.A. (2014). Preservation of mitochondrial functional integrity in mitochondria isolated from small cryopreserved mouse brain areas. Anal. Biochem. 444: 25-31. Doi: 10.1016/j.ab.2013.08.030
Van Gorkom, G.N.Y., Lookermans, E.L., van Elssen, C.H.M.J., Bos, G.M.J. (2019). The effect of vitamin C (ascorbic acid) in the treatment of patients with cancer: A systematic review. Nutrients 11 (5): 977. Doi: 10.3390/nu11050977
Verrax, J., Stockis, J., Tison, A., Taper, H.S., Calderon, P.B. (2006). Oxidative stress by ascorbate/menadione association kills K562 human chronic myelogenous leukaemia cells and inhibits its tumour growth in nude mice. Biochem. Pharmacol. 72 (6): 671-680. Doi: 10.1016/j.bcp.2006.05.025
Xia, J., Xu, H., Zhang, X., Allamargot, C., Coleman, K.L., Nessler, R., Frech, I., Tricot, G., Zhan, F. (2017). Multiple myeloma tumor cells are selectively killed by pharmacologicallydosed ascorbic acid. EBioMedicine 18: 41-49. Doi: 10.1016/j.ebiom.2017.02.011
Yonetani, T. (1960). Studies on cytochrome oxidase. II. Steady state properties. J. Biol. Chem. 235(11): 3138-3143.
Zasowska-Nowak, A., Nowak, P.J., Ciałkowska-Rysz, A. (2021). High-dose vitamin C in advanced-stage cancer patients. Nutrients. 13 (3): 735. Doi: 10.3390/nu13030735
Zhou, J., Chen, C., Chen, X., Fei, Y., Jiang, L., Wang, G. (2020). Vitamin C promotes apoptosis and cell cycle arrest in oral squamous cell carcinoma. Front Oncol. 10: 976. Doi: 10.3389/fonc.2020.00976

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Derechos de autor 2021 Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales