Resumen
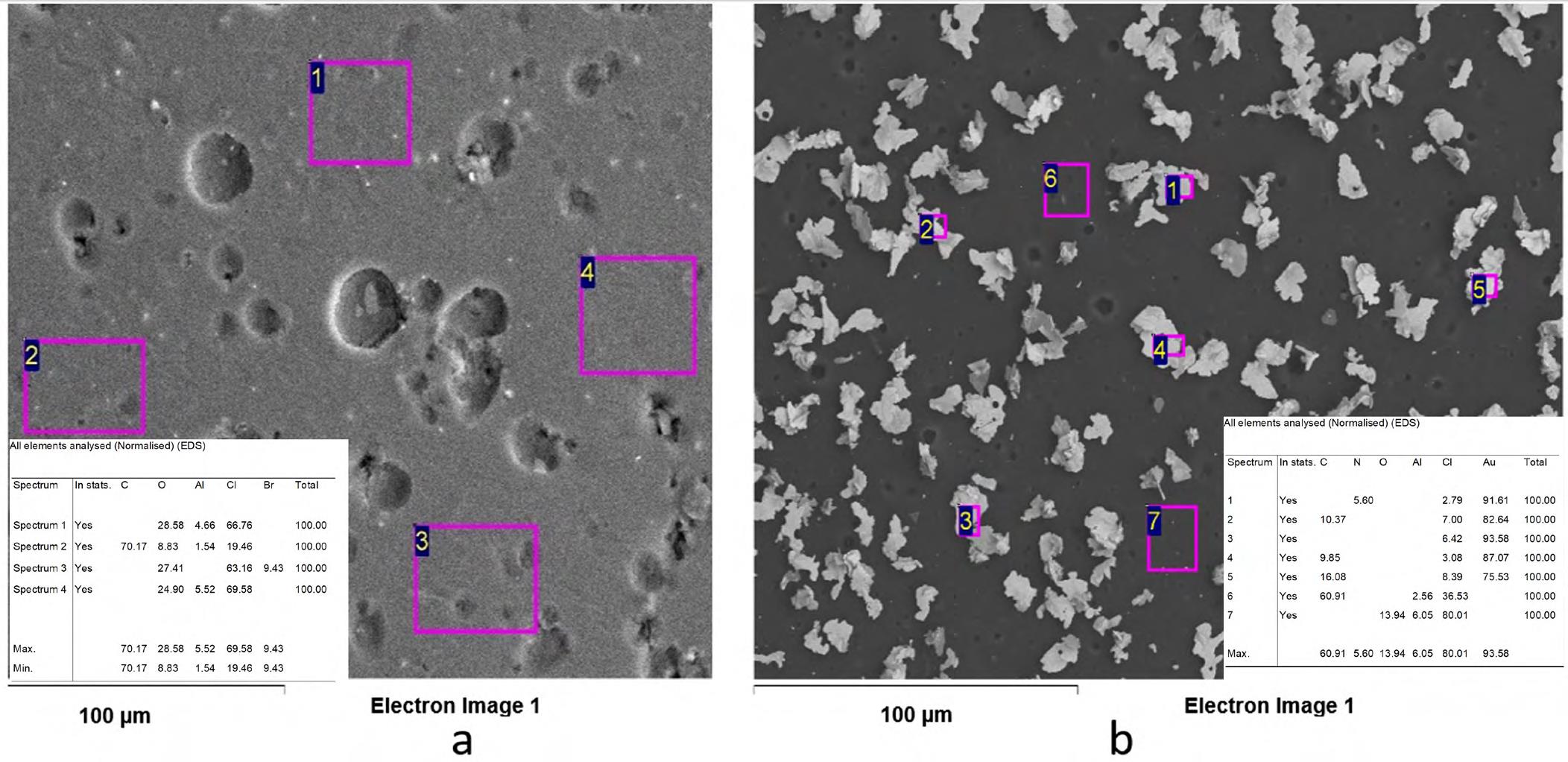
Se sintetizó un nuevo polímero orgánico con características que le permiten actuar como agente extractante de iones metálicos. Se fabricaron membranas a partir de policloruro de vinilo (PVC), plastificante éter de 2 nitrofenil octilo (NPOE) y polímero sintetizado que se emplearon en la extracción de oro a partir de disoluciones acuosas del metal. Se estudió, además, el efecto de la adición de tetrafenilborato de sodio (Na-TFB) para mejorar el intercambio iónico entre la solución de trabajo y la membrana fabricada. Se determinó la composición y el espesor de la membrana con la cual se obtuvo el mayor porcentaje de extracción de oro, así como la selectividad del material hacia Au3+ en presencia de otros iones metálicos (Cu2+, Pb2+, Ca2+, Zn2+, Fe3+, Ni2+ y Al3+), cada uno con una concentración inicial de 1,4x10-4 M. Los resultados mostraron que en un ciclo se alcanzaron porcentajes de extracción cercanos al 80 % del contenido inicial de la solución (1x 10-4 M), con la ventaja de que se pudo reutilizar la misma membrana en tres ciclos sin pérdidas apreciables de eficiencia.
Referencias
Ata, N., Yazicigil, Z., Oztekin, Y. (2008). The electrochemical investigation of salts partition with ion exchange membranes. Journal of Hazardous Materials. 160 (1): 154-160. Doi: 10.1016/j.jhazmat.2008.02.099
Atia, A. A. (2005). Adsorption of silver(I) and gold(III) on resins derived from bisthiourea and application to retrieval of silver ions from processed photo films. Hydrometallurgy. 80 (1-2): 98-106. Doi: 10.1016/J.HYDROMET.2005.07.004
Benavente, J., Oleinikova, M., Muñoz, M., Valiente, M. (1998). Characterization of novel activated composite membranes by impedance spectroscopy. Journal of Electroanalytical Chemistry. 451 (1-2): 173-180. Doi: 10.1016/S0022-0728(98)00070-9
Braibant, B., Bourgeois, D., Meyer, D. (2018). Three-liquid-phase extraction in metal recovery from complex mixtures. Separation and Purification Technology. 195: 367-376. Doi: 10.1016/J.SEPPUR.2017.12.036
Fotoohi, B. & Mercier, L. (2015). Recovery of precious metals from ammoniacal thiosulfate solutions by hybrid mesoporous silica: 2 - A prospect of PGM adsorption. Separation and Purification Technology. 149: 82-91. Doi: 10.1016/j.seppur.2015.05.020
Fu, F. & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: a review. Journal of Environmental Management. 92 (3): 407-418. Doi: 10.1016/j.jenvman.2010.11.011
Gohil, G. S., Binsu, V. V, Shahi, V. K. (2006). Preparation and characterization of mono-valention selective polypyrrole composite ion-exchange membranes. Journal of Membrane Science. 280 (1-2): 210-218. Doi: 10.1016/j.memsci.2006.01.020
Hilson, G. & Monhemius, A. J. (2006). Alternatives to cyanide in the gold mining industry: what prospects for the future? Journal of Cleaner Production. 14 (12-13): 1158-1167. Doi: 10.1016/J.JCLEPRO.2004.09.005
Hosseini, S. M., Madaeni, S. S., Khodabakhshi, A. R. (2010). Heterogeneous cation exchange membrane: preparation, characterization and comparison of transport properties of mono and bivalent cations. Separation Science and Technology. 45 (16): 2308-2321. Doi: 10.1080/01496395.2010.497792
Judd, S. J. (2017). Membrane technology costs and me. Water Research. 122: 1-9. Doi: 10.1016/j.watres.2017.05.027
Kubota, F., Kono, R., Yoshida, W., Sharaf, M., Kolev, S. D., Goto, M. (2019). Recovery of gold ions from discarded mobile phone leachate by solvent extraction and polymer inclusion membrane (PIM) based separation using an amic acid extractant. Separation and Purification Technology. 214: 156-161. Doi: 10.1016/j.seppur.2018.04.031
Li, J. & Miller, J. (2002). Reaction kinetics for gold dissolution in acid thiourea solution using formamidine disulfide as oxidant. Hydrometallurgy. 63 (3): 215-223. Doi: 10.1016/S0304-386X(01)00212-2
Monier, M., Akl, M. A., Ali, W. M. (2014). Modification and characterization of cellulose cotton fibers for fast extraction of some precious metal ions. International Journal of Biological Macromolecules. 66: 125-134. Doi: 10.1016/j.ijbiomac.2014.01.068
Mora-Tamez, L., Rodríguez de San Miguel, E., Briones-Guerash, U., Munguía-Acevedo, N. M., de Gyves, J. (2014). Semi-interpenetrating hybrid membranes containing ADOGEN® 364 for Cd(II) transport from HCl media. Journal of Hazardous Materials: 280: 603-611. Doi: 10.1016/j.jhazmat.2014.08.056
Song, J., Huang, T., Qiu, H., Niu, X., Li, X. M., Xie, Y., He, T. (2018). A critical review on membrane extraction with improved stability: Potential application for recycling metals from city mine. Desalination. 440: 18-38. Doi: 10.1016/j.desal.2018.01.007
Syed, S. (2012). Recovery of gold from secondary sources-A review. Hydrometallurgy. 115-116: 30-51. Doi: 10.1016/j.hydromet.2011.12.012
Tan, P., Jiang, H. R., Zhu, X. B., An, L., Jung, C. Y., Wu, M. C., … Zhao, T. S. (2017). Advances and challenges in lithium-air batteries. Applied Energy. 204: 780-806. Doi: 10.1016/J.APENERGY.2017.07.054
Tofan, L., Bunia, I., Paduraru, C., Teodosiu, C. (2017). Synthesis, characterization and experimental assessment of a novel functionalized macroporous acrylic copolymer for gold separation from wastewater. Process Safety and Environmental Protection. 106 (2): 150-162. Doi: 10.1016/j.psep.2017.01.002
Villalobos, L. F., Yapici, T., Peinemann, K. V. (2014). Poly-thiosemicarbazide membrane for gold recovery. Separation and Purification Technology. 136: 94-104. Doi: 10.1016/j.seppur.2014.08.027
Wang, D. Q., Zhu, M. L., Xuan, F. Z. (2017). Correlation of local strain with microstructures around fusion zone of a Cr-Ni-Mo-V steel welded joint. Materials Science and Engineering A. 685: 205-212. Doi: 10.1016/j.msea.2017.01.015
Zhang, J., Shen, S., Cheng, Y., Lan, H., Hu, X., Wang, F. (2014). Dual lixiviant leaching process for extraction and recovery of gold from ores at room temperature. Hydrometallurgy. 144-145: 114-123. Doi: 10.1016/j.hydromet.2014.02.001

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Derechos de autor 2020 Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales