Resumen
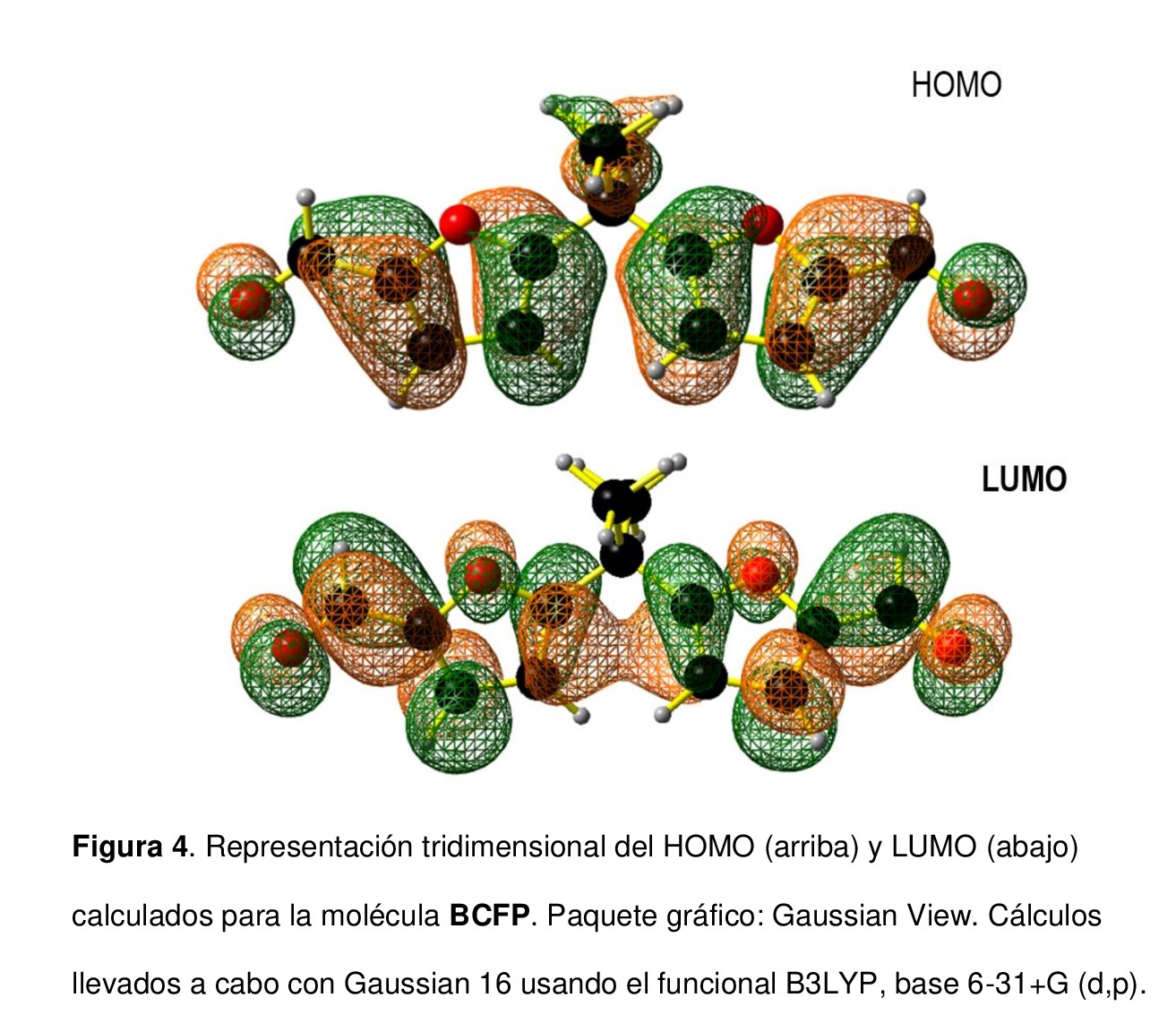
En este estudio se reporta la síntesis y caracterización del compuesto 2,2-bis[5´-(2´-carbonilfuranil)] propano (BCFP), el cual es un prototipo de bloque de construcción para la preparación de macrociclos, polímeros e, incluso, estructuras supramoleculares con diferentes aplicaciones. La síntesis del BCFP se llevó a cabo a través de la reacción de Vilsmeier-Haack del 2,2-bis(2´-furanil) propano usando N,N-dimetilformamida (DMF) y cloruro de fosforilo (POCl3), con un 55 % de rendimiento. El compuesto exhibió una transición nπ* característica de compuestos con átomos sp2 que contienen pares libres no enlazantes. Mediante voltamperometría cíclica se evidenciaron dos procesos redox (reducción y oxidación) de naturaleza electroquímica y química irreversible. Los cálculos teóricos basados en la teoría del funcional de la densidad (density functional theory, DFT) corroboraron las transiciones electrónicas y los procesos redox. Los orbitales moleculares de frontera (HOMO y LUMO) fueron dominados por los grupos aldehído y los anillos de furano.
Referencias
Chen, Z.-H. & Wu, C.-T. (2002). Synthesis of Schiff’s base macrocyclic compounds containing furan ring. Chinese Journal of Organic Chemistry. 22 (8): 582-586.
da Silva, J. L., Beloumini, M. A., Stradiotto, N. R. (2017). Cathodic electrochemical determination of furfural in sugarcane bagasse using an electrode modified with nickel nanoparticles. Analytical Methods. 9 (5): 826-834.
Eseyin, A. E. & Steele, P. H. (2015). An overview of the applications of furfural and its derivatives. International Journal of Advanced Chemistry. 3 (2): 42-47.
Fauque, L. L. J. (1954). U.S. Patent 2,681,917.
Frisch, N. M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Fox, D. J. (2016). Gaussian 16, Revision B.01, Gaussian, Inc., Wallingford CT.
Gaitonde, V., Lee, K., Kirschbaum, K., Sucheck, S. J. (2014). Bio-based bisfuran: synthesis, crystal structure, and low molecular weight amorphous polyester. Tetrahedron Letters. 55: 4141-4145.
Gandini, A. & Belgacem, M. N. (1997). Furans in polymer chemistry. Progress in Polymer Science. 22 (7): 1203-1379.
Hoydonckx, H. E., Van Rhijn, W. M., Van Rhijn, W., De Vos, D. E., Jacobs, P. A. (2007). Furfural and Derivatives. En Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim, Alemania: Wiley-VCH. Doi: 10.1002/14356007.a12_119.pub2
Katritzky, A.R., Ramsden, C.A., Joule, J.A., Zhdankin, V.V. 3.3 - Reactivity of Five-Membered Rings with One Heteroatom, Editor(s): Alan R.
Katritzky, Christopher A. Ramsden, John A. Joule, Viktor V. Zhdankin. Handbook of Heterocyclic Chemistry (Third Edition), Elsevier, 2010, p. 383-472, ISBN 9780080958439. Doi: 10.1016/B978-0-08-095843-9.00009-4
Limpricht, H. (1870). Ueber das Tetraphenol C4H4O. Berichte der Deutschen Chemischen Gesellschaft.3 (1): 90-91.
Mariscal, R., Maireles-Torres, P., Ojeda, M., Sádaba, I., López-Granados, M. (2016). Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy & Environmental Science. 9 (4): 1144-1189.
Moore, J. & Kelly, J. E. (1978). Polyesters Derived from Furan and Tetrahydrofuran Nuclei. Macromolecules. 11 (3): 568-573.
Moore, J. & Kelly, J. E. (1978). Thermally initiated crosslinking of an unsaturated heterocyclic polyester. Journal of Polymer Science Part A: Polymer Chemistry. 16 (9): 2407-2409.
Moore, J. & Kelly, J. E. (1979). Polymerization of furandicarbonyl chloride with bisphenol A poly (2,5-furandiylcarbonyloxy-1,4-phenylenedimethylmethylene-1,4-phenyleneoxycarbonyl). Polymer. 20 (5): 627-628.
Moore, J. & Kelly, J. E. (1984). Polyhydroxymethylfuroate [poly(2,5‐furandiylcarbonyloxy methylene)]. Journal of Polymer Science Part A: Polymer Chemistry. 22 (3): 863-864.
Peters, F. N. Jr. (1939). Industrial Uses of Furans. Industrial and Engineer Chemistry. 31 (2): 178-180.
Vilsmeier, A. & Haack, A. (1927). Über die Einwirkung von Halogenphosphor auf Alkyl‐formanilide. Eine neue Methode zur Darstellung sekundärer und tertiärer p‐Alkylamino‐benzaldehyde.
Berichte der Deutschen Chemischen Gesellschaft zu Berlin. 60 (1): 119-122.
Zeng, C., Seino, H., Ren, J., Hatanaka, K., Yoshie, N. (2013). Bio-Based Furan Polymers with Self-Healing Ability. Macromolecules. 46 (5): 1794-1802.

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Derechos de autor 2021 Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales