Resumen
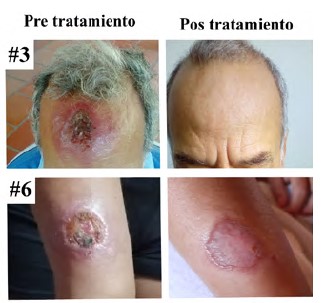
Ante la necesidad de desarrollar nuevos medicamentos para el tratamiento de la leishmaniasis cutánea que sean efectivos, seguros, de mayor aceptación entre los pacientes, fáciles de administrar en los entornos rurales y más económicos, en el presente estudio se evaluó in vitro e in vivo el potencial terapéutico y el perfil de citotoxicidad de un extracto etanólico y un extracto glicólico de las ramas de Caesalpinia spinosa (Molina) Kuntze y sus respectivas formulaciones: loción y emulsión. Asimismo, se validó la utilidad de las dos formulaciones como tratamiento tópico en diez pacientes con leishmaniasis cutánea no complicada. Ambos extractos fueron activos contra Leishmania braziliensis en concentraciones de menos de 22 μg/mL, tuvieron baja toxicidad en las células U-937 y HL60 cuando se administraron en forma pura y en las células Hep G2 y Detroit 551 cuando se administraron en concentraciones de menos de 20 %. Ni los extractos ni las formulaciones fueron irritantes o corrosivos y no causaron ningún otro tipo de toxicidad dérmica. La exposición de fibroblastos al extracto glicólico y a las formulaciones favoreció su migración y la reparación de la monocapa luego de 16 horas de contacto, permitiendo cierres de la brecha entre 33,9 y 70,9 %. Tanto los extractos como las formulaciones favorecieron la curación completa de los hámsteres con leishmaniasis cutánea en porcentajes mayores al 80 %. La loción y la emulsión se absorben y penetran en la piel después de su aplicación y, aunque se alcanzan a detectar en plasma hasta las 24 h (la loción) o las 6 h (la emulsión) de la aplicación, la mayor parte queda retenida en la piel. Por último, el tratamiento de los diez pacientes con la loción y la emulsión logró la curación completa de las lesiones con la reparación de la piel dañada y sin efectos adversos. En conclusión, los resultados demostraron las propiedades leishmanicidas y cicatrizantes de C. spinosa, lo que le permitiría ser una alternativa segura para el tratamiento local de la leishmaniasis cutánea. Es necesario validar estos resultados en ensayos clínicos controlados y determinar la eficacia y la seguridad del tratamiento con los productos fitoterapéuticos de C. spinosa.
Referencias
Alvar, J., Yactayo, S., Bern, C. (2006). Leishmaniasis and poverty. Trends in Parasitol. 22: 552-556.
Alvar, J., Vélez., I.D., Bern, C., Herrero, M., Desjeux, P., Cano, J., Jannin, J., den Boer, M., WHO Leishmaniasis Control Team. (2012). Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 7: 35671
Ben-Salah, A., Ben-Messaoud, N., Guedri, E., Zaatour, A., Ben-Alaya., N, Bettaieb, J., Gharbi, A., Belhadj-Hamida, N., Boukthir, A., Chlif, S., Abdelhamid K., El Ahmadi, Z., Louzir, H., Mokni, M., Morizot, G., Buffet, P., Smith, P.L., Kopydlowski, K.M., Kreishman Deitrick, M., Smith, K.S., Nielsen, C.J., Ullman, D.R., Norwood, J.A., Thorne, G.D., McCarthy, W.F., Adams, R.C., Rice, R.M., Tang, D., Berman, J., Ransom, J., Magill, A.J., Grogl, M. (2013). Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N Engl J Med. 368: 524-32. doi: 10.1056/NEJMoa1202657.
Bennis, I., Thys, S., Filali, H., De Brouwere, V., Sahibi, H., Boelaert, M. (2017). Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. 6: 146. Doi: 10.1186/s40249-017-0267-5
Cardona-Arias, J.A., Patiño-Martínez, D.A., López-Carvajal, L. (2017a). Evaluaciones económicas en leishmaniasis cutánea: revisión sistemática de literatura 1980-2015. Revista de Economía del Caribe. 20: 52-70.
Cardona-Arias, J.A., López-Carvajal, L., Tamayo Plata, M.P., Vélez I.D. (2017b). Costeffectiveness analysis of thermotherapy versus pentavalent antimonials for the treatment of cutaneous leishmaniasis. J Evid Based Med. 10: 81-90. Doi: 10.1111/jebm.12245.
Cardona-Arias, J.A., López-Carvajal, L., Tamayo-Plata, M.P., Vélez, I.D. (2018). Comprehensive economic evaluation of thermotherapy for the treatment of cutaneous leishmaniasis in Colombia. BMC Public Health. 18: 185. Doi: 10.1186/s12889-018-5060-2
Castañeda DM, Pombo LM, Urueña CP, Hernández JF, Fiorentino S. (2012). A gallotannin-rich fraction from Caesalpinia spinosa (Molina) Kuntze displays cytotoxic activity and raises sensitivity to doxorubicin in a leukemia cell line. BMC Complement Altern Med. 12: 38. Doi: 10.1186/1472-6882-12-38
De la Cruz-Lapa, P. (2004). Aprovechamiento integral y racional de la tara. Revista del Instituto de Investigación FIGMMG. 7: 64-73.
De la Cruz, H., Vilcapoma, G., Zevallos P.A. (2007). Ethnobotanical study of medicinal plants used by the Andean people of Canta, Lima, Perú. J Ethnopharmacol. 111: 284-94.
Hotez, P. (2013). Forgotten People, Forgotten Diseases: The Neglected Tropical Diseases and Their Impact on Global Health and Development, 2nd ed.; ASM Press: Washington, DC, USA.
Kloucek, P., Polesny, Z., Svobodova, B., Vlkova, E., Kokoska, L. (2005). Antibacterial screening of some Peruvian medicinal plants used in Callería District. J Ethnopharmacol. 99: 309-312. Doi: 10.1016/j.jep.2005.01.062
Kondo, K., Takaishi, Y., Shibata, H., Higuti, T. (2006). ILSMRs (intensifier of beta-lactamsusceptibility in methicillin-resistant Staphylococcus aureus) from Tara [Caesalpinia spinosa (Molina) Kuntze]. Phytomed. 13: 209-212. Doi: 10.1016/j.phymed.2004.08.001
Ladopoulos, T., Ntais, P., Tsirigotakis, N., Dokianakis, E., Antoniou, M. (2015). The proliferation potential of promastigotes of the main Leishmania species of the old world in NNN culture medium prepared using blood of four different mammals. Exp Parasitol. 157: 124‐127. Doi:10.1016/j.exppara.2015.07.008
López, L., Vélez, I., Asela, C., Cruz, C., Alves, F., Robledo, S., Arana, B. (2018). A phase II study to evaluate the safety and efficacy of topical 3% amphotericin B cream (Anfoleish) for the treatment of uncomplicated cutaneous leishmaniasis in Colombia. PLoS Negl Trop Dis. 12:e0006653. Doi: 10.1371/journal.pntd.0006653.
Montalvo, A.M., Fraga, J., Montano, I., Monzote, L., Van der Auwera, G., Marín, M., Muskus, C. (2016). Molecular identification of Leishmania spp. clinical isolates from Colombia based on hsp70 gene. Biomédica. 36: 37-44. Doi: 10.7705/biomedica.v36i2.2688.
Montoya, A., Daza, A., Muñoz, D., Ríos, K., Taylor, V., Cedeño, D., Vélez, I.D., Echeverri, F., Robledo, S.M. (2015). Development
f a novel formulation with hypericin to treat cutaneous leishmaniasis based on photodynamic therapy in in vitro and in vivo studies. Antimicrob Agents Chemother. 59: 5804-5813. Doi: 10.1128/AAC.00545-15
Narváez, A., Calvo, A., Troya, A.M. (2009). Las poblaciones naturales de la tara (Caesalpinia spinosa) en el Ecuador: una aproximación al conocimiento de la diversidad genética y el contenido de taninos a través de estudios moleculares y bioquímicos. Serie Investigación y Sistematización No. 7. Programa Regional Ecobona-Intercooperation, Laboratorio de Biotecnología Vegetal Escuela de Ciencias Biológicas Pontificia Universidad Católica del Ecuador PUCE. Quito. 39 pp.
OECD. (2004), Test No. 427: Skin Absorption: In Vivo Method, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. https://doi.org/10.1787/9789264071063-en
OECD. (2017), Test No. 402: Acute Dermal Toxicity, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. https://doi.org/10.1787/9789264070585-en
OECD. (2019a). Test No. 431: In vitro skin corrosion: Reconstructed human epidermis (RHE) test method, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. https://doi.org/10.1787/9789264264618-en
OECD. (2019b). Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. https://doi.org/10.1787/9789264242845-en
Oryan, A. (2015). Plant-derived compounds in treatment of leishmaniasis. Iran J Vet Res. 16: 1-19.
Parvizi, M. M., Handjani, F., Moein, M., Hatam, G., Nimrouzi, M., Hassanzadeh, J., Hamidizadeh, N., Khorrami, H. R., Zarshenas, M. M. (2017). Efficacy of cryotherapy plus topical Juniperus excelsa M. Bieb cream versus cryotherapy plus placebo in the treatment of Old World cutaneous leishmaniasis: A triple-blind randomized controlled clinical trial. PLoS Negl Trop Dis. 11:e0005957. https://doi.org/10.1371/journal.pntd.0005957
Pulido, S.A., Muñoz, D.L., Restrepo, A.M., Mesa, C.V, Alzate, J.F., Vélez, I.D., Robledo, S.M. (2012). Improvement of the green fluorescent protein reporter system in Leishmania spp. for the in vitro and in vivo screening of antileishmanial drugs. Acta Trop. 122: 36-45. Doi: 10.1016/j.actatropica.2011.11.015
Rangel-Ch., J.O. (2015). La biodiversidad de Colombia: significado y distribución regional. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 39: 176-200. Doi: http://dx.doi.org/10.18257/raccefyn.136
Rigano, L., Deola, M., Zaccariotto, F., Colleoni, T., Lionetti, N. (2019). A New Gelling Agent and Rheology Modifier in Cosmetics: Caesalpinia spinosa Gum. Cosmetics. 6: 34.
Robledo S.M., Carrillo, L.M., Daza, A., Restrepo, A.M., Muñoz, D.L., Tobón, J., Murillo, J.D., López, A., Ríos, C., Mesa, C.V., Upegui, Y.A., Valencia-T., A., Mondragón-Sh, K., Rodríguez, B., Vélez, I.D. (2012). Cutaneous leishmaniasis in the dorsal skin of hamsters: A useful model for the screening of antileishmanial drugs. J Vis Exp. pii: 3533. Doi: 10.3791/3533
Robledo, M.B, Robledo, S.M., Ceballos, A.L., Vélez, I.D., Gómez, N., Zuluaga, J.J. (2018). Formulación del producto crema aceite en agua útil para aplicación tópica en mucosas y piel contra enfermedades producidas por parásitos, hongos y bacterias. Resolución No. 32414 de mayo 11 de 2018.
Santander, S. P., Aoki, M., Hernández, J. F., Pombo, M., Moins-Teisserenc, H., Mooney, N., Fiorentino, S. (2011). Galactomannan from Caesalpinia spinosa induces phenotypic and functional maturation of human dendritic cells. Int Immunopharmacol. 11: 652-660. Doi: 10.1016/j.intimp.2011.01.006
Sen, R., Chatterjee, M. (2011). Plant derived therapeutics for the treatment of leishmaniasis. Phytomed. 18: 1056-69. Doi: 10.1016/j.phymed.2011.03.004
Sosa, N., Capitán, Z., Nieto, J., Nieto, M., Calzada, J., Paz, H., Spadafora, C., Kreishman- Deitrick, M., Kopydlowski, K., Ullman, D., McCarthy, W.F., Ransom, J., Berman, J., Scott, C., Grogl, M. (2013). Randomized, double-blinded, phase 2 trial of WR 279,396 (paromomycin and gentamicin) for cutaneous leishmaniasis in Panama. Am J Trop Med Hyg. 89: 557-563. Doi: 10.4269/ajtmh.12-0736
Sosa, N., Pascale, J.M., Jiménez, A.I., Norwood, J.A., Kreishman-Detrick, M., Weina, P.J., Lawrence, K., McCarthy, W.F., Adams, R.C., Scott, C., Ransom, J., Tang, D., Grogl, M. (2019). Topical paromomycin for New World cutaneous leishmaniasis. PLoS Negl Trop Dis. 13 (5): e0007253. Doi: 10.1371/journal.pntd.0007253
Soto, J., Soto, P., Ajata, A., Luque, C., Tintaya, C., Paz D, Rivero D., Berman, J. (2019). Topical 15% Paromomycin-Aquaphilic for Bolivian Leishmania braziliensis Cutaneous Leishmaniasis: A Randomized, Placebo-controlled Trial. Clin Infect Dis. 68: 844-849. Doi: 10.1093/cid/ciy619
Skowyra, M. Falguera, V., Gallego, G., Peiró, S., Almajano, M. del P. (2014). Antioxidant properties of aqueous and ethanolic extracts of tara (Caesalpinia spinosa) pods in vitro and in model food emulsions. J Sci Food Agric. 94: 911-918.
Trouiller, P., Olliaro, P., Torreele, E., Orbinski, J., Laing, R., Ford, N. (2002). Drug development for neglected diseases: A deficient market and a publicontrol negativohealth policy failure. Lancet. 359: 2188-2194.
Upegui, Y., Ríos, K., Quiñones, W., Echeverri, F., Archbold, R., Murillo, J.D., Torres, F., Escobar, G., Vélez, I.D., Robledo, S.M. (2019). Chroman-4-one hydrazones derivatives: Synthesis, characterization, and in vitro and in vivo antileishmanial effects. Med Chem Res. 28: 2184-2199 (2019). Doi: 10.1007/s00044-019-02446-x
Urueña, C., Mancipe, J., Hernández, J., Castañeda, D., Pombo, L., Gómez, A., Asea, A., Fiorentino, S. (2013). Gallotannin-rich Caesalpinia spinosa fraction decreases the primary tumor and factors associated with poor prognosis in a murine breast cancer model. BMC Complement Altern Med. 13: 74. Doi.org/10.1186/1472-6882-13-74
The United States Pharmacopeia; the national formulary. (2016). 39a. rev. (USP39) 34a. ed. (NF34). Rockville, Md.: United States Pharmacopeial Convention, c2016.
Vélez, I.D., Robledo, S.M., Robledo, M.B., Ceballos, A.L., Giraldo, N.A., Gómez, A., Zuluaga, J.J. (2017). Cream formulation with amphotericin B and oil in water useful for topical application to mucous tissue and skin against diseases produced by leishmaniasis. US9801895B1.
World Health Organization. (2010). Control of the leishmaniasis: Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22-26 March 2010.WHO Technical Report Series 949.
Zerehsaz, F., Beheshti, S., Reza Rezaian, G., Joubeh, S. (2003). Erysipeloid cutaneous leishmaniasis: Treatment with a new, topical, pure herbal extract. Eur J Dermatol. 13: 145-148.

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Derechos de autor 2020 Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales