Resumen
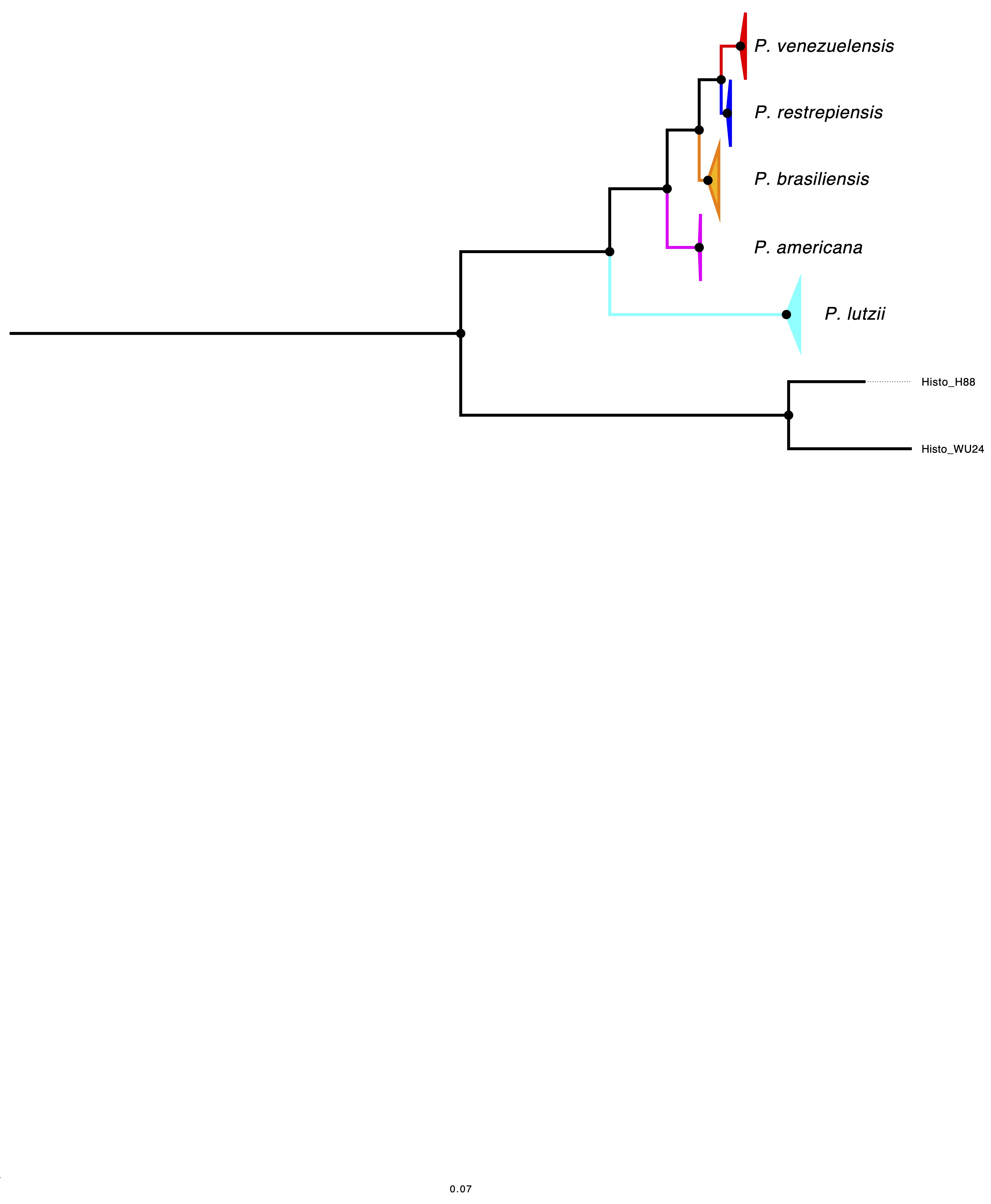
El género Paracoccidioides hoy abarca cinco especies: P. lutzii, P. brasiliensis sensu stricto, P. americana, P. venezuelensis y P. restrepiensis. Todos los casos de paracoccidioidomicosis reportados en Colombia han sido causados por P. restrepiensis, denominado así en honor de Ángela Restrepo, líder en el estudio de este hongo. Las evaluaciones previas de la diversidad genética han sugerido que las especies de Paracoccidioides difieren en su polimorfismo. Para inferir cambios en el tamaño efectivo de la población, se utilizó el método del coalescente markoviano secuencial por pares (pairwise sequentially Markovian coalescent, PSMC) mediante la generación de dos pseudodiploides para P. restrepiensis y uno para P. brasiliensis sensu stricto. Se encontró que P. restrepiensis divergió de sus especies hermanas recientemente, con un tiempo de divergencia en relación con P. venezuelensis de 125.000 (± 42.000) años. Los análisis que utilizan el PSMC muestran una reducción sistemática en el tamaño efectivo de la población de P. restrepiensis, con una rápida disminución de la variabilidad genética en comparación con P. brasiliensis sensu stricto, lo que indica que P. restrepiensis se ha visto expuesto a un cuello de botella poblacional sistemático. Ninguna de las otras dos especies muestra la drástica reducción efectiva del tamaño de la población observada en P. restrepiensis. Estas comparaciones sugieren que la trayectoria de P. restrepiensis es, de alguna manera, diferente de las otras especies de Paracoccidioides y llevan a preguntarse cuáles han sido los eventos biogeográficos que han producido cambios tan dramáticos en el patrón poblacional.
Referencias
Almeida, F. P. (1930). Estudos comparativos do granuloma coccidióidico nos Estados Unidos e no Brasil. Novo gênero para o parasito brasileiro. Annals Faculdade de Medicina Sao Paulo 5, 125-141.
Assolini, J. P., Lenhard-Vidal, A., Bredt, C. S. O., Tano, Z. N., Sano, A., Cezar-Dos-Santos, F., Ono, M. A., Itano, E. N. (2021). Distinct Pattern of Paracoccidioides lutzii, P. restrepiensis and P. americana Antigens Recognized by IgE in Human Paracoccidioidomycosis. Current Microbiology, 78(7), 2608-2614. https://doi.org/10.1007/s00284-021-02508-7
Bagagli, E., Matute, D. R., Garces, H. G., Tenorio, B. G., Garces, A. G., Alves, L. G. B., Yamauchi, D. H., Hrycyk, M. F., Barker, B. M., Teixeira, M. M. (2021). Paracoccidioides brasiliensis Isolated from Nine-Banded Armadillos (Dasypus novemcinctus) Reveal Population Structure and Admixture in the Amazon Basin. Journal of Fungi (Basel), 7(1). https://doi.org/10.3390/jof7010054
Bazzicalupo, E., Lucena-Pérez, M., Kleinman-Ruiz, D., Pavlov, A., Trajçe, A., Hoxha, B., Sanaja, B., Gurielidze, Z., Kerdikoshvili, N., Mamuchadze, J., Yarovenko, Y. A., Akkiev, M. I., Ratkiewicz, M., Saveljev, A. P., Melovski, D., Gavashelishvili, A., Schmidt, K., Godoy, J. A. (2022). History, demography and genetic status of Balkan and Caucasian Lynx lynx (Linnaeus, 1758) populations revealed by genome-wide variation. Diversity and Distributions, 28(1), 65-82. https://doi.org/https://doi.org/10.1111/ddi.13439
Bellissimo-Rodrigues, F., Machado, A. A., Martinez, R. (2011). Paracoccidioidomycosis epidemiological features of a 1,000-cases series from a hyperendemic area on the southeast of Brazil. The American Journal of Tropical Medicine and Hygiene, 85(3), 546-550. https://doi.org/10.4269/ajtmh.2011.11-0084
Cocio, T.A., Nascimento, E., Kress, M., Bagagli, E., Martinez, R. (2020). Characterization of a Paracoccidioides spp. strain from southeastern Brazil genotyped as Paracoccidioides restrepiensis (PS3) and review of this phylogenetic species. Genetics and Molecular Biology, 43(2), e20190201. https://doi.org/10.1590/1678-4685-GMB-2019-0201
Coutinho, Z.F., Wanke, B., Travassos, C., Oliveira, R.M., Xavier, D.R., Coimbra, C. E., Jr. (2015). Hospital morbidity due to paracoccidioidomycosis in Brazil (1998-2006). Tropical Medicine and Intternational Health, 20(5), 673-680. https://doi.org/10.1111/tmi.12472
Dagilis, A.J., Peede, D., Coughlan, J.M., Jofre, G.I., D’Agostino, E.R.R., Mavengere, H., Tate, A.D., Matute, D.R. (2022). A need for standardized reporting of introgression: Insights from studies across eukaryotes. Evolution Letters, n/a(n/a). https://doi.org/https://doi.org/10.1002/evl3.294
DePristo, M.A., Banks, E., Poplin, R., Garimella, K.V., Maguire, J.R., Hartl, C., Philippakis, A.A., del Angel, G., Rivas, M.A., Hanna, M., McKenna, A., Fennell, T.J., Kernytsky, A.M., Sivachenko, A.Y., Cibulskis, K., Gabriel, S.B., Altshuler, D., Daly, M.J. (2011). A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nature Genetics, 43(5), 491-498. https://doi.org/10.1038/ng.806
Desjardins, C.A., Champion, M.D., Holder, J.W., Muszewska, A., Goldberg, J., Bailao, A.M., Brigido, M.M., Ferreira, M.E., Garcia, A.M., Grynberg, M., Gujja, S., Heiman, D.I., Henn, M.R., Kodira, C.D., Leon-Narvaez, H., Longo, L.V., Ma, L.J., Malavazi, I., Matsuo, A.L., . . . Cuomo, C. A. (2011). Comparative genomic analysis of human fungal pathogens causing paracoccidioidomycosis. PLoS Genetics, 7(10), e1002345. https://doi.org/10.1371/journal.pgen.1002345
Farlow, A., Long, H., Arnoux, S., Sung, W., Doak, T.G., Nordborg, M., Lynch, M. (2015). The Spontaneous Mutation Rate in the Fission Yeast Schizosaccharomyces pombe. Genetics, 201(2), 737-744. https://doi.org/10.1534/genetics.115.177329
Franco, M.F., Del Negro, G., Lacaz, C.d.S., Restrepo-Moreno, A. (1994). Paracoccidioidomycosis. CRC Press. Gao, Z., Przeworski, M., Sella, G. (2015). Footprints of ancient-balanced polymorphisms in genetic variation data from closely related species. Evolution, 69(2), 431-446. https://doi.org/10.1111/evo.12567
Guerrero, R.F., Hahn, M.W. (2018). Quantifying the risk of hemiplasy in phylogenetic inference. Proceedings of the National Academy of Sciences USA, 115(50), 12787-12792. https://doi.org/10.1073/pnas.1811268115
Hamlin, J.A.P., Hibbins, M.S., Moyle, L.C. (2020). Assessing biological factors affecting postspeciation introgression. Evolution Letters, 4(2), 137-154. https://doi.org/10.1002/evl3.159
Lacaz Cda, S., Vidal, M.S., Pereira, C.N., Heins-Vaccari, E.M., de Melo, N.T., Sakai-Valente, N., Arriagada, G.L. (1997). Paracoccidioides cerebriformis Moore, 1935. Mycologic and immunochemical study. Rev Inst Med Trop Sao Paulo, 39(3), 141-144. https://doi.org/10.1590/s0036-46651997000300003
Leffler, E.M., Bullaughey, K., Matute, D.R., Meyer, W.K., Segurel, L., Venkat, A., Andolfatto, P., Przeworski, M. (2012). Revisiting an old riddle: what determines genetic diversity levels within species? PLoS Biology, 10(9), e1001388. https://doi.org/10.1371/journal.pbio.1001388
Li, H., Durbin, R. (2011). Inference of human population history from individual whole-genome sequences. Nature, 475(7357), 493-496. https://doi.org/10.1038/nature10231
Lutz, A. (1908). Uma mycose pseudo-coccidica localizada na boca e observada no Brazil: contribuicao ao conhecimento das hyphoblastomycoses americanas. Brasil Medico, 22, 121-124, 141-144.
Lutz, A. (2004). Lutz A Obra completa Uma micose pseudococcídica localizada na boca e observada no Brasil. Contribuição ao conhecimento das hifoblastomicoses americanas.
Fiocruz Lynch, M., Sung, W., Morris, K., Coffey, N., Landry, C.R., Dopman, E. B., Dickinson, W.J., Okamoto, K., Kulkarni, S., Hartl, D.L., Thomas, W.K. (2008). A genome-wide view of the spectrum of spontaneous mutations in yeast. Proceedings of the National Academy of Sciences USA, 105(27), 9272-9277. https://doi.org/10.1073. pnas.0803466105
Mattos, K., Cocio, T. A., Chaves, E. G. A., Borges, C.L., Venturini, J., de Carvalho, L.R., Mendes, R.P., Paniago, A.M.M., Weber, S.S. (2021). An update on the occurrence of Paracoccidioides species in the Midwest region, Brazil: Molecular epidemiology, clinical aspects and serological profile of patients from Mato Grosso do Sul State. PLoS Neglected Tropical Diseases, 15(4), e0009317. https://doi.org/10.1371/journal.pntd.0009317
Matute, D.R., McEwen, J.G., Puccia, R., Montes, B.A., San-Blas, G., Bagagli, E., Rauscher, J.T., Restrepo, A., Morais, F., Nino-Vega, G., Taylor, J.W. (2006). Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Molecular Biology and Evolution, 23(1), 65-73. https://doi.org/10.1093/molbev/msj008
Matute, D.R., Sepulveda, V.E. (2019). Fungal species boundaries in the genomics era. Fungal Genetics and Biology, 131, 103249. https://doi.org/10.1016/j.fgb.2019.103249
Matute, D.R., Sepulveda, V.E., Quesada, L.M., Goldman, G.H., Taylor, J.W., Restrepo, A., McEwen, J.G. (2006). Microsatellite analysis of three phylogenetic species of Paracoccidioides brasiliensis. Journal of Clinical Microbiology, 44(6), 2153-2157. https://doi.org/10.1128/JCM.02540-05
Matute, D.R., Torres, I.P., Salgado-Salazar, C., Restrepo, A., McEwen, J.G. (2007). Background selection at the chitin synthase II (chs2) locus in Paracoccidioides brasiliensis species complex. Fungal Genetics and Biology, 44(5), 357-367. https://doi.org/10.1016/j.fgb.2007.01.006
Mavengere, H., Mattox, K., Teixeira, M.M., Sepulveda, V.E., Gomez, O.M., Hernandez, O., McEwen, J., Matute, D.R. (2020). Paracoccidioides Genomes Reflect High Levels of Species Divergence and Little Interspecific Gene Flow. mBio, 11(6), e01999-01920. https://doi.org/10.1128/mBio.01999-20
Maxwell, C.S., Mattox, K., Turissini, D.A., Teixeira, M.M., Barker, B.M., Matute, D.R. (2019). Gene exchange between two divergent species of the fungal human pathogen, Coccidioides. Evolution, 73(1), 42-58. https://doi.org/10.1111/evo.13643
McKenna, A., Hanna, M., Banks, E., Sivachenko, A., Cibulskis, K., Kernytsky, A., Garimella, K., Altshuler, D., Gabriel, S., Daly, M., DePristo, M.A. (2010). The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research, 20(9), 1297-1303. https://doi.org/10.1101/gr.107524.110
Morais, F.V., Barros, T.F., Fukada, M.K., Cisalpino, P.S., Puccia, R. (2000). Polymorphism in the gene coding for the immunodominant antigen gp43 from the pathogenic fungus paracoccidioides brasiliensis. Journal of Clinical Microbiology, 38(11), 3960-3966. http://jcm.asm.org/cgi/content/abstract/38/11/3960
Muñoz, J.F., Farrer, R.A., Desjardins, C.A., Gallo, J.E., Sykes, S., Sakthikumar, S., Misas, E., Whiston, E.A., Bagagli, E., Soares, C.M., Teixeira, M.M., Taylor, J. W., Clay, O.K., McEwen, J.G., Cuomo, C.A. (2016). Genome Diversity, Recombination, and Virulence across the Major Lineages of Paracoccidioides. mSphere, 1(5), e00213-00216. https://doi.org/10.1128/mSphere.00213-16
Niño-Vega, G.A., Calcagno, A.M., San-Blas, G., San-Blas, F., Gooday, G.W., & Gow, N.A. (2000). RFLP analysis reveals marked geographical isolation between strains of Paracoccidioides brasiliensis. Medical Mycology, 38(6), 437-441. https://doi.org/10.1080/mmy.38.6.437.441
PAHO, (1971). Paracoccidioidomycosis. Proceedings of the First Pan American Symposium, Medellin, Colombia, Medellín, Colombia.
Restrepo, A. (2000). Morphological aspects of Paracoccidioides brasiliensis in lymph nodes: implications for the prolonged latency of paracoccidioidomycosis?. Medical Mycology, 38(4), 317-322. https://doi.org/10.1080/mmy.38.4.317.322
Restrepo, A., Arango, M. D. (1980). In vitro susceptibility testing of Paracoccidioides brasiliensis to sulfonamides. Antimicrobial Agents and Chemotherapy, 18(1), 190-194. http://www.ncbi.nlm.nih.gov/pubmed/7416744
Restrepo-Moreno, A., Tobon-Orozco, A.M., González-Marín, A. (2020). Paracoccidioidomycosis. In J. E. Bennett, R. Dolin, & M. J. Blaser (Eds.), Mandell, Douglas, and Bennett’s principles and practice of infectious diseases (Ninth edition. ed., Vol. 2, pp. 3211-3221).Elsevier.
Sato, Y., Ogden, R., Kishida, T., Nakajima, N., Maeda, T., Inoue-Murayama, M. (2020). Population history of the golden eagle inferred from whole-genome sequencing of three of its subspecies. Biological Journal of the Linnean Society, 130(4), 826-838. https://doi.org/10.1093/biolinnean/blaa068
Schön, I., Martens, K., Dijk, P.V. (2009). Lost sex: the evolutionary biology of parthenogenesis. Springer.
Scorzoni, L., de Lucas, M.P., Singulani, J.L., de Oliveira, H.C., Assato, P.A., Fusco-Almeida, A.M., Mendes-Giannini, M.J.S. (2018). Evaluation of Caenorhabditis elegans as a host model for Paracoccidioides brasiliensis and Paracoccidioides lutzii. Pathogens and Disease, 76(1). https://doi.org/10.1093/femspd/fty004
Siqueira, I.M., Fraga, C.L., Amaral, A.C., Souza, A.C., Jeronimo, M.S., Correa, J.R., Magalhaes, K.G., Inacio, C.A., Ribeiro, A.M., Burguel, P.H., Felipe, M.S., Tavares, A.H., Bocca, A.L. (2016). Distinct patterns of yeast cell morphology and host responses induced by representative strains of Paracoccidioides brasiliensis (Pb18) and Paracoccidioides lutzii (Pb01). Medical Mycology, 54(2), 177-188. https://doi.org/10.1093/mmy/myv072
Soares, C., Madlun, E., da Silva, S., Pereira, M., Felipe, M. (1995). Characterization of Paracoccidioides brasiliensis isolates by random amplified polymorphic DNA analysis. Journal of Clinical Microbiology, 33(2), 505-507. https://doi.org/10.1128/jcm.33.2.505-507.1995
Splendore, A. (1912). Zymonematosi con localizzazione nella cavita della bocca, osservata in Brasile. Bulletin de la Société de Pathologie Exotique, 5, 313–319.
Taylor, J. W., Branco, S., Gao, C., Hann-Soden, C., Montoya, L., Sylvain, I., Gladieux, P. (2017). Sources of Fungal Genetic Variation and Associating It with Phenotypic Diversity. Microbiology Spectrum, 5(5). https://doi.org/10.1128/microbiolspec.FUNK-0057-2016
Teixeira Mde, M., Theodoro, R.C., Oliveira, F.F., Machado, G.C., Hahn, R.C., Bagagli, E., San-Blas, G., Soares Felipe, M.S. (2014). Paracoccidioides lutzii sp. nov.: biological and clinical implications. Medical Mycology, 52(1), 19-28. https://doi.org/10.3109/13693786.2013.794311
Teixeira, M.M., Cattana, M.E., Matute, D.R., Munoz, J.F., Arechavala, A., Isbell, K., Schipper, R., Santiso, G., Tracogna, F., Sosa, M. L. A., Cech, N., Alvarado, P., Barreto, L., Chacon, Y., Ortellado, J., Lima, C. M., Chang, M. R., Nino-Vega, G., Yasuda, M.A.S., . . . Giusiano, G. (2020). Genomic diversity of the human pathogen Paracoccidioides across the South American continent. Fungal Genetics and Biology, 140, 103395. https://doi.org/10.1016/j.fgb.2020.103395
Teixeira, M.M., Theodoro, R.C., de Carvalho, M. J., Fernandes, L., Paes, H.C., Hahn, R.C., Mendoza, L., Bagagli, E., San-Blas, G., Felipe, M.S. (2009). Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus [Research Support, Non-U.S. Gov’t]. Mol Phylogenet Evol, 52(2), 273-283. https://doi.org/10.1016/j.ympev.2009.04.005
Teixeira, M.M., Theodoro, R.C., Nino-Vega, G., Bagagli, E., Felipe, M.S. (2014). Paracoccidioides species complex: ecology, phylogeny, sexual reproduction, and virulence. PLoS Pathogens, 10(10), e1004397. https://doi.org/10.1371/journal.ppat.1004397
Turissini, D.A., Gómez, O.M., Teixeira, M.M., McEwen, J.G., Matute, D.R. (2017). Species boundaries in the human pathogen Paracoccidioides. Fungal Genetics and Biology, 106, 9-25. https://doi.org/10.1016/j.fgb.2017.05.007

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Derechos de autor 2022 Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales