Resumen
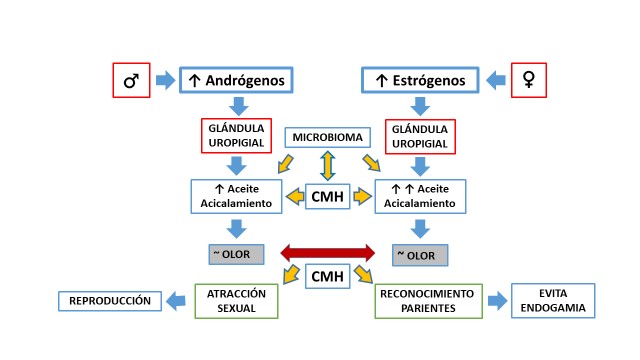
La selección sexual desempeña un papel evolutivo fundamental en muchas especies. En las aves la selección intersexual la realizan principalmente las hembras durante el periodo reproductivo al escoger a los machos para aparearse y transmitir sus genes a la descendencia. Los ornamentos, más notorios en este periodo, se han propuesto como señal de calidad, pero no es claro cómo se asocian con otros caracteres más directamente relacionados con la supervivencia, que permiten reaccionar contra los patógenos, por ejemplo, la respuesta inmune, específicamente el complejo mayor de histocompatibilidad (CMH). Hay evidencia de que el olor del aceite de acicalamiento está asociado al CMH y permite determinar similitudes y diferencias entre parejas. Esta revisión presenta un panorama de las investigaciones de las últimas décadas sobre el papel del olor y el CMH en la selección intersexual en aves. Revisamos el estado del conocimiento sobre el CMH y sus productos en aves no paseriformes, que tienen una estructura genética del CMH muy simple, y paseriformes, que presentan múltiples duplicaciones de los genes de este complejo. Asimismo, revisamos los reportes que sustentan la asociación entre el CMH y la selección de parejas, particularmente en hembras que buscan parejas sexuales diferentes que aumentan la heterocigocidad y la variedad del CMH en la descendencia y disminuyen la homocigocidad y la endogamia. Aún existen grandes lagunas en el conocimiento de la selección sexual en muchas aves, que requieren una aproximación interdisciplinaria para comprender plenamente los mecanismos y el significado de este fascinante proceso evolutivo.
Referencias
Abbas, A K., Litchamn, A H., Pillai, S. (2018). Celliular and molecular Immunology. Elsevier Inc.
Abplanalp, H., Sato, K., Napolitano, D., Reid, J. (1992). Reproductive Performance of Inbred Congenic Leghorns Carrying Different Haplotypes for the Major Histocompatibility Complex. Poultry Science, 71(1), 9-17. [https://doi.org/10.3382/ps.0710009](https://doi.org/10.3382/ps.0710009)
Alves Soares, T., Caspers, B. A., Loos H. M. (2024). Volatile organic compounds in preen oil and feathers – a review. Biologial Reviews. [https://doi.org/10.1111/brv.13059](https://doi.org/10.1111/brv.13059)
Amo, L., Amo-de Paz, G., Kabbert, J., Machordom, A. (2022). House sparrows do not exhibit a preference for the scent of potential partners with different MHC-I diversity and genetic distances. PLOS ONE, 17(12), e0278892. [https://doi.org/10.1371/journal.pone.0278892](https://doi.org/10.1371/journal.pone.0278892)
Amo, L., López-Rull, I. (2023). Zebra Finch Females Can Assess Male Quality via Olfaction. Preprints. [https://www.preprints.org/manuscript/202305.2241/v1](https://www.preprints.org/manuscript/202305.2241/v1)
Arct, A., Drobniak, S. M., Podmokła, E., Gustafson, L., Cichoń, M. (2013). Benefits of extra-pair mating may depend on environmental conditions—an experimental study in the blue tit (Cyanistes caeruleus). Behavioral Ecology and Sociobiology, 67(11), 1809-1815. [https://doi.org/10.1007/s00265-013-1588-4](https://doi.org/10.1007/s00265-013-1588-4)
Ardia, D. R. (2005). Cross-fostering reveals an effect of spleen size and nest temperatures on immune responses in nestling European starlings. Oecologia, 145(2), 326-333. [https://doi.org/10.1007/s00442-005-0120-6](https://doi.org/10.1007/s00442-005-0120-6)
Balakrishnan, C. N., Ekblom, R., Völker, M., Westerdahl, H., Godinez, R., Kotkiewicz, H., Burt, DW., Graves, T., Griffin, DK., Warren, WC., Edwards, S. V. (2010). Gene duplication and fragmentation in the zebra finch major histocompatibility complex. BMC Biology, 8(1), 29. [https://doi.org/10.1186/1741-7007-8-29](https://doi.org/10.1186/1741-7007-8-29)
Bethanne-Zelano, Scott V., Edwards. (2002). An MHC Component to Kin Recognition and Mate Choice in Birds: Predictions, Progress, and Prospects. The American Naturalist, 160(S6), S225-S237. [https://doi.org/10.1086/342897](https://doi.org/10.1086/342897)
Bjorkman, P. J. (2006). Finding the groove. Nature Immunology, 7(8), 787-789. [https://doi.org/10.1038/ni0806-787](https://doi.org/10.1038/ni0806-787)
Bollmer, J. L., Dunn, P. O., Whittingham, L. A., Wimpee, C. (2010). Extensive MHC class II B gene duplication in a passerine, the common yellowthroat (Geothlypis trichas). Journal of Heredity, 101(4), 448-460. [https://doi.org/10.1093/jhered/esq018](https://doi.org/10.1093/jhered/esq018)
Bonadonna, F., Sanz-Aguilar, A. (2012). Kin recognition and inbreeding avoidance in wild birds: the first evidence for individual kin-related odour recognition. Animal Behaviour, 84(3), 509-513. [https://doi.org/10.1016/j.anbehav.2012.06.014](https://doi.org/10.1016/j.anbehav.2012.06.014)
Bonforte R. J., Topilsky M., Siltzbach L. E., Glade P. R. (1972). Phytohemagglutinin skin test: a possible in vivo measure of cell-mediated immunity. Journal of Pediatrics, 81(4), 775-781. [https://doi.org/10.1016/S0022-3476(72)80101-X](https://doi.org/10.1016/S0022-3476(72)80101-X)
Bonneaud, C., Chastel, O., Federici, P., Westerdahl, H., Sorci, G. (2006). Complex MHC-based mate choice in a wild passerine. Proceedings of the Royal Society B: Biological Sciences, 273 (1590), 1111-1116. https://doi.org/10.1098/rspb.2005.3325
Bonneaud, C., Mazuc, J., Chastel, O., Westerdahl, H., Sorci, G. (2004). Terminal Investment Induced by Immune Challenge and Fitness Traits Associated with Major Histocompatibility Complex in the House Sparrow. Evolution, 58(12), 2823-2830. [http://www.jstor.org/stable/3449435](http://www.jstor.org/stable/3449435)
Bonneaud, C., Mazuc, J., González, G., Haussy, C., Chastel, O., Faivre, B., Sorci, G. (2003). Assessing the cost of mounting an immune response. The American Naturalist, 161(3), 367-379. [https://doi.org/10.1086/346134](https://doi.org/10.1086/346134)
Bonneaud, C., Sorci, G., Morin, V., Westerdahl, H., Zoorob, R., Wittzell, H. (2004). Diversity of MHC class I and IIB genes in house sparrows (Passer domesticus). Immunogenetics, 55(12), 855-865. [https://doi.org/10.1007/s00251-004-0648-3](https://doi.org/10.1007/s00251-004-0648-3)
Boyse, E. A. (1986). HLA and the chemical senses. Human Immunology, 15(4), 391-395. [https://doi.org/10.1016/0198-8859(86)90016-9](https://doi.org/10.1016/0198-8859(86)90016-9)
Briles, W., McGibbon, W., Irwin, M. (1950). On multiple alleles effecting cellular antigens in the chicken. Genetics, 35(6), 633. [https://eurekamag.com/research/013/778/013778886.php](https://eurekamag.com/research/013/778/013778886.php)
Briles, W. E., Stone, H. A., Cole, R. K. (1977). Marek’s Disease: Effects of B Histocompatibility Alloalleles in Resistant and Susceptible Chicken Lines. Science, 195(4274), 193-195. [https://doi.org/10.1126/science.831269](https://doi.org/10.1126/science.831269)
Caspers, B., Gagliardo, A., Krause, E. T. (2015). Impact of kin odour on reproduction in zebra finches. Behavioral Ecology and Sociobiology, 69, 1827-1833. [https://doi.org/10.1007/s00265-015-1995-9](https://doi.org/10.1007/s00265-015-1995-9)
Colegrave, N., Kotiaho, J. S.,Tomkins, J. L. (2002). Mate choice or polyandry: reconciling genetic compatibility and good genes sexual selection. Evolutionary Ecology Research, 4, 911-917. [https://www.semanticscholar.org/paper/Mate-choice-or-polyandry%3A-reconciling-geneticand-Colegrave-Kotiaho/12a5ca143204d2597b4bd69985efe4d09cca6b0b](https://www.semanticscholar.org/paper/Mate-choice-or-polyandry%3A-reconciling-geneticand-Colegrave-Kotiaho/12a5ca143204d2597b4bd69985efe4d09cca6b0b)
Cooper, M. D., Peterson, R. D. A., Good, R. A. (1965). Delineation of the Thymic and Bursal Lymphoid Systems in the Chicken. Nature, 205(4967), 143-146. [https://doi.org/10.1038/205143a0](https://doi.org/10.1038/205143a0)
D’Urban-Jackson, J., dos Remedios, N., Maher, K. H., Zefania, S., Haig, S., Oyler-McCance, S., Blomqvist, D., Burke, T., Bruford, M.W., Székely, T., Küpper, C. (2017). Polygamy slows down population divergence in shorebirds. Evolution, 71(5), 1313-1326. [https://doi.org/10.1111/evo.13212](https://doi.org/10.1111/evo.13212)
Dandine-Roulland, C., Laurent, R., Dall’Ara, I., Toupance, B., Chaix, R. (2019). Genomic evidence for MHC disassortative mating in humans. Proceedings of the Royal Society B: Biological Sciences, 286(1899), 20182664. [https://doi.org/10.1098/rspb.2018.2664](https://doi.org/10.1098/rspb.2018.2664)
Daniel, J. Y., Vignon, F., Assenmacher, I., Rochefort, H. (1977). Evidence of androgen and estrogen receptors in the preen gland of male ducks. Steroids, 30(5), 703-709. [https://doi.org/10.1016/0039-128x(77)90059-9](https://doi.org/10.1016/0039-128x(77)90059-9)
Delany, M., Robinson, C., Goto, R., Miller, M. (2009). Architecture and Organization of Chicken Microchromosome 16: Order of the NOR, MHC-Y, and MHC-B Subregions. The Journal of Heredity, 100, 507-514. [https://doi.org/10.1093/jhered/esp044](https://doi.org/10.1093/jhered/esp044)
Drews, A., Strandh, M., Råberg, L.,Westerdahl, H. (2017). Expression and phylogenetic analyses reveal paralogous lineages of putatively classical and non-classical MHC-I genes in three sparrow species (Passer). BMC Evolutionary Biology, 17, 152. [https://doi.org/10.1186/s12862-017-0970-7](https://doi.org/10.1186/s12862-017-0970-7)
Drews, A., Westerdahl, H. (2019). Not all birds have a single dominantly expressed MHC-I gene: Transcription suggests that siskins have many highly expressed MHC-I genes. Scientific Reports, 9(1), 19506. https://doi.org/10.1038/s41598-019-55800-9
Folstad, I., Karter, A. J. (1992). Parasites, bright males, and the immunocompetence handicap. The American Naturalist, 139(3), 603-622.
Foo, Y. Z., Rhodes, G., Simmons, L. (2016). The effects of sex hormones on immune function: A meta-analysis. Biological reviews of the Cambridge Philosophical Society, 92, 551-571. [https://doi.org/10.1111/brv.12243](https://doi.org/10.1111/brv.12243)
Fossøy, F., Johnsen, A., Lifjeld, J. T. (2008). Multiple genetic benefits of female promiscuity in a socially monogamous passerine. Evolution, 62(1), 145-156. [https://doi.org/10.1111/j.1558-5646.2007.00284.x](https://doi.org/10.1111/j.1558-5646.2007.00284.x)
Freeman-Gallant, C., Meguerdichian, M., Wheelwright, N., Sollecito, S. (2003). Social pairing and female mating fidelity predicted by restriction fragment length polymorphism similarity at the major histocompatibility complex in a songbird. Molecular Ecology, 12, 3077-3083. [https://doi.org/10.1046/j.1365-294X.2003.01968.x](https://doi.org/10.1046/j.1365-294X.2003.01968.x)
Fulton, J. E., McCarron, A. M., Lund, A. R., Pinegar, K. N., Wolc, A., Chazara, O., Bed’Hom, B., Berres, M., Miller, M. M. (2016). A high-density SNP panel reveals extensive diversity, frequent recombination and multiple recombination hotspots within the chicken major histocompatibility complex B region between BG2 and CD1A1. Genetics Selection Evolution, 48(1), 1. [https://doi.org/10.1186/s12711-015-0181-x](https://doi.org/10.1186/s12711-015-0181-x)
Futuyma, D. J. (2021). How birds evolve: what science reveals about their origins, lives, and diversity. Princeton University Press.
Garvin, J. C., Abroe, B., Pedersen, M. C., Dunn, P. O., Whittingham, L. A. (2006). Immune response of nestling warblers varies with extra-pair paternity and temperature. Molecular Ecology, 15(12), 3833-3840. [https://doi.org/10.1111/j.1365-294X.2006.03042.x](https://doi.org/10.1111/j.1365-294X.2006.03042.x)
Gilles, M., Fokkema, R. W., Korsten, P., Caspers, B. A., Schmoll, T. (2024). Preen oil composition of Pied Flycatchers is similar between partners but differs between sexes and breeding stages. Ibis, 166(1), 171-186. [https://doi.org/10.1111/ibi.13246](https://doi.org/10.1111/ibi.13246)
Gillingham, M. A., Richardson, D. S., Løvlie, H., Moynihan, A., Worley, K., Pizzari, T. (2009). Cryptic preference for MHC-dissimilar females in male red junglefowl, Gallus gallus. Proceedings of the Royal Society B: Biological Sciences, 276(1659), 1083-1092. [https://doi.org/10.1098/rspb.2008.1549](https://doi.org/10.1098/rspb.2008.1549)
Glick, B., Chang, T. S., Jaap, R. G. (1956). The Bursa of Fabricius and Antibody Production. Poultry Science, 35(1), 224-225. [https://doi.org/10.3382/ps.0350224](https://doi.org/10.3382/ps.0350224)
Gohli, J., Anmarkrud, J. A., Johnsen, A., Kleven, O., Borge, T., Lifjeld, J. T. (2013). Female promiscuity is positively associated with neutral and selected genetic diversity in passerine birds. Evolution, 67(5), 1406-1419. [https://doi.org/10.1111/evo.12045](https://doi.org/10.1111/evo.12045)
Gould, S. J. (1985). La Sonrisa del flamenco. Reflexiones sobre historia natural. Traducción Antonio Resinas. Crítica. Barcelona. España ISBN 978-84-8432-564-2
Griffith, S. C., Owens, I. P. F., Thuman, K. A. (2002). Extra pair paternity in birds: a review of interspecific variation and adaptive function. Molecular Ecology, 11(11), 2195-2212. [https://doi.org/10.1046/j.1365-294X.2002.01613.x](https://doi.org/10.1046/j.1365-294X.2002.01613.x)
Hagelin, J. (2009). Bird odors and other chemical substances: A defense mechanism or overlooked mode of intraspecific communication? The Auk, 124, 741-761. [https://doi.org/10.1642/0004-8038(2007)124[741:BOAOCS]2.0.CO;2](https://doi.org/10.1642/0004-8038(2007)124[741:BOAOCS]2.0.CO;2)
Halupka, L., O’Connor, E., Strandh, M., Sztwiertnia, H., Klimczuk, E., Hasselquist, D., Westerdahl, H. (2023). Female reed warblers in social pairs with low MHC dissimilarity achieve higher MHC compatibility through random extra-pair matings. bioRxiv, 2023.2004.2017.537178. [https://doi.org/10.1101/2023.04.17.537178](https://doi.org/10.1101/2023.04.17.537178)
Hamal, K. R., Burgess S., Pevzner I. Y., Erf G. F. (2006). Maternal Antibody Transfer from Dams to Their Egg Yolks, Egg Whites, and Chicks in Meat Lines of Chickens. Poultry Science, 85, 1364-1372. [https://doi.org/10.1093/ps/85.8.1364](https://doi.org/10.1093/ps/85.8.1364)
Hamilton, W. D., Zuk, M. (1982). Heritable True Fitness and Bright Birds: A Role for Parasites? Science, 218(4570), 384-387. [https://doi.org/10.1126/science.7123238](https://doi.org/10.1126/science.7123238)
Hanssen, S. A., Folstad, I., Erikstad, K. E. (2003). Reduced Immunocompetence and Cost of Reproduction in Common Eiders. Oecologia, 136(3), 457-464. [http://www.jstor.org/stable/4223696](http://www.jstor.org/stable/4223696)
Hasselquist, D. (2007). Comparative immunoecology in birds: hypotheses and tests. Journal of Ornithology, 148(2), 571-582. [https://doi.org/10.1007/s10336-007-0201-x](https://doi.org/10.1007/s10336-007-0201-x)
Hasselquist, D., Sherman, P. (2001). Social mating system and extrapair fertilization in passerine birds. Behavioral Ecology, 12, 457-466. [https://doi.org/10.1093/beheco/12.4.457](https://doi.org/10.1093/beheco/12.4.457)
He, K., Liang, C.-h., Zhu, Y., Dunn, P., Zhao, A., Minias, P. (2022). Reconstructing Macroevolutionary Patterns in Avian MHC Architecture With Genomic Data. Frontiers in Genetics, 13. [https://doi.org/10.3389/fgene.2022.823686](https://doi.org/10.3389/fgene.2022.823686)
He, K., Minias, P., Dunn, P. O. (2020). Long-Read Genome Assemblies Reveal Extraordinary Variation in the Number and Structure of MHC Loci in Birds. Genome Biology and Evolution, 13(2), evaa270. [https://doi.org/10.1093/gbe/evaa270](https://doi.org/10.1093/gbe/evaa270)
Hoover, B., Alcaide, M., Jennings, S., Sin, S. Y. W., Edwards, S. V., Nevitt, G. A. (2018). Ecology can inform genetics: Disassortative mating contributes to MHC polymorphism in Leach’s storm-petrels (Oceanodroma leucorhoa). Molecular Ecology, 27(16), 3371-3385. [https://doi.org/10.1111/mec.14801](https://doi.org/10.1111/mec.14801)
Johnsen, A., Andersen, V., Sunding, C., Lifjeld, J. T. (2000). Female bluethroats enhance offspring immunocompetence through extra-pair copulations. Nature, 406(6793), 296-299. [https://doi.org/10.1038/35018556](https://doi.org/10.1038/35018556)
Jones, A. G., & Ratterman, N. L. (2009). Mate choice and sexual selection: What have we learned since Darwin? Proceedings of the National Academy of Sciences, 106(supplement 1), 10001-10008. [https://doi.org/10.1073/pnas.0901129106](https://doi.org/10.1073/pnas.0901129106)
Kamiya, T., O’Dwyer, K., Westerdahl, H., Senior, A., Nakagawa, S. (2014). A quantitative review of MHC-based mating preference: the role of diversity and dissimilarity. Molecular Ecology, 23 (21), 5151-5163. [https://doi.org/10.1111/mec.12934](https://doi.org/10.1111/mec.12934)
Kaufman, J. (2013). Antigen processing and presentation: evolution from a bird’s eye view. Molecular Immunology, 55(2), 159-161. [https://doi.org/10.1016/j.molimm.2012.10.030](https://doi.org/10.1016/j.molimm.2012.10.030)
Kaufman, J., Milne, S., Göbel, T. W. F., Walker, B. A., Jacob, J. P., Auffray, C., Zoorob, R., Beck, S. (1999). The chicken B locus is a minimal essential major histocompatibility complex. Nature, 401(6756), 923-925. [https://doi.org/10.1038/44856](https://doi.org/10.1038/44856)
Klein, J. (1986). Natural History of the Major Histocompatibility Complex. John Wiley & Son.
Koch, M., Camp, S., Collen, T., Avila, D., Salomonsen, J., Wallny, H.-J., van Hateren, A., Hunt, L., Jacob, J. P., Johnston, F., Marston, D. A., Shaw, I., Dunbar, P. R., Cerundolo, V., Jones, E. Y., Kaufman, J. (2007). Structures of an MHC Class I Molecule from B21 Chickens Illustrate Promiscuous Peptide Binding. Immunity, 27(6), 885-899. [https://doi.org/10.1016/j.immuni.2007.11.007](https://doi.org/10.1016/j.immuni.2007.11.007)
Lakshmanan, N., Gavora, J., Lamont, S. (1997). Major Histocompatibility Complex Class II DNA Polymorphisms in Chicken Strains Selected for Marek’s Disease Resistance and Egg Production or for Egg Production Alone. Poultry Science, 76, 1517-1523. [https://doi.org/10.1093/ps/76.11.1517](https://doi.org/10.1093/ps/76.11.1517)
Landry, C., Garant, D., Duchesne, P., Bernatchez, L. (2001). ´Good genes as heterozygosity´: the major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar). Proceedings of the Royal Society of London. Series B: Biological Sciences, 268(1473), 1279-1285. [https://doi.org/10.1098/rspb.2001.1659](https://doi.org/10.1098/rspb.2001.1659)
Leclaire, S., Strandh, M., Mardon, J., Westerdahl, H., Bonadonna, F. (2017). Odour-based discrimination of similarity at the major histocompatibility complex in birds. Proceedings of the Royal Society B: Biological Sciences, 284. [https://doi.org/10.1098/rspb.2016.2466](https://doi.org/10.1098/rspb.2016.2466)
Leclaire, S., van Dongen, W. F. D., Voccia, S., Merkling, T., Ducamp, C., Hatch, S. A., Blanchard, P., Danchin, E., Wagner, R. H. (2014). Preen secretions encode information on MHC similarity in certain sex-dyads in a monogamous seabird. Scientific Reports, 4(1), 6920. [https://doi.org/10.1038/srep06920](https://doi.org/10.1038/srep06920)
Lifjeld, J. T., Gohli, J., Albrecht, T., Garcia-del-Rey, E., Johannessen, L. E., Kleven, O., Marki, P. Z., Omotoriogun, T. C., Rowe, M., Johnsen, A. (2019). Evolution of female promiscuity in Passerides songbirds. BMC Evolutionary Biology, 19(1), 169. [https://doi.org/10.1186/s12862-019-1493-1](https://doi.org/10.1186/s12862-019-1493-1)
Lifjeld, J. T., & Robertson, R. J. (1992). Female control of extra-pair fertilization in tree swallows. Behavioral Ecology and Sociobiology, 31(2), 89-96. [https://doi.org/10.1007/BF00166341](https://doi.org/10.1007/BF00166341)
Lindsay, W. R., Andersson, S., Bererhi, B., Höglund, J., Johnsen, A., Kvarnemo, C., Leder, E. H., Lifjeld, J. T., Ninnes, C. E., Olsson, M., Parker, G. A., Pizzari, T., Qvarnström, A., Safran, R. J., Svensson, O., Edwards, S. V. (2019). Endless forms of sexual selection. PeerJ, 7, e7988. [https://doi.org/10.7717/peerj.7988](https://doi.org/10.7717/peerj.7988)
Lovlie, H., Gillingham, M., Worley, K., Pizzari, T., Richardson, D. (2013). Cryptic female choice favours sperm from major histo-compaibility complex-dissimilar males. Proceedings. Biological sciences / The Royal Society, 280, 20131296. [https://doi.org/10.1098/rspb.2013.1296](https://doi.org/10.1098/rspb.2013.1296)
Mellinger, S., Stervander, M., Lundberg, M., Drews, A., Westerdahl, H. (2023). Improved haplotype resolution of highly duplicated MHC genes in a long-read genome assembly using MiSeq amplicons. PeerJ, 11, e15480. [https://doi.org/10.7717/peerj.15480](https://doi.org/10.7717/peerj.15480)
Merha N.K. (Ed) (2010). The HLA complex in biology and medicine. A resource book. New Delhi, India. Jaypee Brothers Medical Publishers (p) Ltd.
Mihailova, M., Berg, M. L., Buchanan, K. L., Bennett, A.T.D. (2014). Odour-based discrimination of subspecies, species and sexes in an avian species complex, the crimson rosella. Animal Behaviour, 95, 155-164. [https://doi.org/10.1016/j.anbehav.2014.07.012](https://doi.org/10.1016/j.anbehav.2014.07.012)
Miller, M. M., Goto, R., Bernot, A., Zoorob, R., Auffray, C., Bumstead, N., Briles, W. E. (1994). Two Mhc class I and two Mhc class II genes map to the chicken Rfp-Y system outside the B complex. Proceedings of the National Academy of Sciences, 91(10), 4397-4401. [https://doi.org/10.1073/pnas.91.10.4397](https://doi.org/10.1073/pnas.91.10.4397)
Miller, M. M., Robinson, C. M., Abernathy, J., Goto, R. M., Hamilton, M. K., Zhou, H., Delany, M. E. (2013). Mapping Genes to Chicken Microchromosome 16 and Discovery of Olfactory and Scavenger Receptor Genes Near the Major Histocompatibility Complex. Journal of Heredity, 105(2), 203-215. [https://doi.org/10.1093/jhered/est091](https://doi.org/10.1093/jhered/est091)
Miller, M. M., Taylor, R. L. (2016). Brief review of the chicken Major Histocompatibility Complex: the genes, their distribution on chromosome 16, and their contributions to disease resistance. Poultry Science, 95(2), 375-392. [https://doi.org/10.3382/ps/pev379](https://doi.org/10.3382/ps/pev379)
Minias, P., He, K., Dunn, P. O. (2021). The strength of selection is consistent across both domains of the MHC class I peptide-binding groove in birds. BMC Ecology and Evolution, 21(1), 80. [https://doi.org/10.1186/s12862-021-01812-x](https://doi.org/10.1186/s12862-021-01812-x)
Minias, P., Pikus, E., Whittingham, L. A., Dunn, P. O. (2018a). Evolution of Copy Number at the MHC Varies across the Avian Tree of Life. Genome Biology and Evolution, 11(1), 17-28. [https://doi.org/10.1093/gbe/evy253](https://doi.org/10.1093/gbe/evy253)
Minias, P., Pikus, E., Whittingham, L. A., Dunn, P. O. (2018b). A global analysis of selection at the avian MHC. Evolution, 72(6), 1278-1293. [https://doi.org/10.1111/evo.13490](https://doi.org/10.1111/evo.13490)
Minias, P., Whittingham, L. A., Dunn, P. O. (2017). Coloniality and migration are related to selection on MHC genes in birds. Evolution, 71(2), 432-441.
Mougeot, F., Irvine, J. R., Seivwright, L., Redpath, S. M., Piertney, S. (2004). Testosterone, immunocompetence, and honest sexual signaling in male red grouse. Behavioral Ecology, 15(6), 930-937. [https://doi.org/10.1093/beheco/arh087](https://doi.org/10.1093/beheco/arh087)
O’Connor, E. A., Westerdahl, H., Burri, R., Edwards, S. V. (2019). Avian MHC Evolution in the Era of Genomics: Phase 1.0. Cells, 8(10), 1152. [https://doi.org/10.3390/cells8101152](https://doi.org/10.3390/cells8101152)
Painter, C. A. & Stern, L. J. (2012). Conformational variation in structures of classical and non-classical MHCII proteins and functional implications. Immunological Reviews, 250(1), 144-157. [https://doi.org/10.1111/imr.12003](https://doi.org/10.1111/imr.12003)
Penn, D. (2002). The Scent of Genetic Compatibility: Sexual Selection and the Major Histocompatibility Complex. Ethology, 108, 1-21. [https://doi.org/10.1046/j.1439-0310.2002.00768.x](https://doi.org/10.1046/j.1439-0310.2002.00768.x)
Penn, D. & Potts, W. (1998). MHC-disassortative mating preferences reversed by cross-fostering. Proceedings Biological Society, 265(1403), 1299-1306.
Penn, D. J. & Potts, W. K. (1999). The Evolution of Mating Preferences and Major Histocompatibility Complex Genes. The American Naturalist, 153(2), 145-164. [https://doi.org/10.1086/303166](https://doi.org/10.1086/303166)
Peters, A., Astheimer, L. B., Boland, C. R. J., Cockburn, A. (2000). Testosterone is involved in acquisition and maintenance of sexually selected male plumage in superb fairy-wrens, Malurus cyaneus. Behavioral Ecology and Sociobiology, 47(6), 438-445. [https://doi.org/10.1007/s002650050688](https://doi.org/10.1007/s002650050688)
Petrie, M. & Kempenaers, B. (1998). Extra-pair paternity in birds: explaining variation between species and populations. Trends in Ecology & Evolution, 13(2), 52-58. [https://doi.org/10.1016/S0169-5347(97)01232-9](https://doi.org/10.1016/S0169-5347(97)01232-9)
Pikus, E., Dunn, P. O., Minias, P. (2022). High MHC diversity confers no advantage for phenotypic quality and reproductive performance in a wild bird. Journal of Animal Ecology, 91(8), 1707-1718. [https://doi.org/10.1111/1365-2656.13737](https://doi.org/10.1111/1365-2656.13737)
Pineaux, M., Blanchard, P., Ribeiro, L., Hatch, S. A., Leclaire, S. (2023). A Gull Species Recognizes MHC-II Diversity and Dissimilarity Using Odor Cues. Paper presented at the Chemical Signals in Vertebrates 15. [https://hal.science/hal-03918940](https://hal.science/hal-03918940)
Pink, J. R. L., Droege, W., Hála, K., Miggiano, V. C., Ziegler, A. (1977). A three-locus model for the chicken major histocompatibility complex. Immunogenetics, 5(1), 203-216. [https://doi.org/10.1007/BF01570477](https://doi.org/10.1007/BF01570477)
Potts, W. K. & Wakeland, E. K. (1993). Evolution of MHC genetic diversity: a tale of incest, pestilence and sexual preference. Trends in Genetics, 9(12), 408-412. [https://doi.org/10.1016/0168-9525(93)90103-O](https://doi.org/10.1016/0168-9525(93)90103-O)
Rekdal, S. L., Anmarkrud, J. A., Lifjeld, J. T., Johnsen, A. (2023). Do female bluethroats without extra-pair offspring have more MHC-compatible social mates? Behavioral Ecology and Sociobiology, 77(3), 36. [https://doi.org/10.1007/s00265-023-03311-z](https://doi.org/10.1007/s00265-023-03311-z)
Ren, L., Yang, Z., Wang, T., Sun, Y., Guo, Y., Zhang, Z., Fei, J., Bao, Y., Qin, T., Wang, J., Huang, Y., Hu, X., Zhao, Y., Li, N. (2011). Characterization of the MHC class II α-chain gene in ducks. Immunogenetics, 63 (10), 667-678. https://doi.org/10.1007/s00251-011-0545-5
Ribatti, D., Crivellato, E., Vacca, A. (2006). The contribution of Bruce Glick to the definition of the role played by the bursa of Fabricius in the development of the B cell lineage. Clinical and Experimental Immunology, 145(1), 1-4.
Richardson, D. S., Komdeur, J., Burke, T., Von Schantz, T. (2005). MHC-based patterns of social and extra-pair mate choice in the Seychelles warbler. Proceedings of the Royal Society B: Biological Sciences, 272(1564), 759-767. [https://doi.org/10.1098/rspb.2004.3028](https://doi.org/10.1098/rspb.2004.3028).
Ridley, M. (2000). Mendel´s Demon: Gene justice and the complexity of life. Phoenix.
Roved, J., Westerdahl, H., Hasselquist, D. (2017). Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Hormones and Behavior, 88, 95-105. [https://doi.org/10.1016/j.yhbeh.2016.11.017](https://doi.org/10.1016/j.yhbeh.2016.11.017)
Saino, N., Romano, M., Rubolini, D., Caprioli, M., Costanzo, A., Canova, L., Møller, A. P. (2014). Melanic coloration differentially predicts transfer of immune factors to eggs with daughters or sons. Behavioral Ecology, 25(5), 1248-1255. [https://doi.org/10.1093/beheco/aru112](https://doi.org/10.1093/beheco/aru112)
Sallaberry-Pincheira, N., González-Acuña, D., Padilla, P., Dantas, G. P. M., Luna-Jorquera, G., Frere, E., Valdés-Velásquez, A., Vianna, J. A. (2016). Contrasting patterns of selection between MHC I and II across populations of Humboldt and Magellanic penguins. Ecology and Evolution, 6(20), 7498-7510. [https://doi.org/10.1002/ece3.2502](https://doi.org/10.1002/ece3.2502)
Salomonsen, J., Marston, D., Avila, D., Bumstead, N., Johansson, B., Juul-Madsen, H., Olensen, G.D., Riegert P., Skjødt, K., Vainio O., Wiles, M.V., Kaufman, J. (2003). The properties of the single chicken MHC classical class II α chain (B-LA) gene indicate an ancient origin for the DR/E-like isotype of class II molecules. Immunogenetics, 55(9), 605-614. [https://doi.org/10.1007/s00251-003-0620-7](https://doi.org/10.1007/s00251-003-0620-7)
Sato, K., Abplanalp, H., Napolitano, D., Reid, J. (1992). Effects of Heterozygosity of Major Histocompatibility Complex Haplotypes on Performance of Leghorn Hens Sharing a Common Inbred Background. Poultry Science, 71(1), 18-26. [https://doi.org/10.3382/ps.0710018](https://doi.org/10.3382/ps.0710018)
Schat, K. A. (1994). Cell-mediated immune effector functions in chickens. Poultry Science, 73(7), 1077-1081. [https://doi.org/10.3382/ps.0731077](https://doi.org/10.3382/ps.0731077)
Schierman, L. W. & Nordskog, A. W. (1961). Relationship of Blood Type to Histocompatibility in Chickens. Science, 134(3484), 1008-1009. [https://doi.org/10.1126/science.134.3484.1008](https://doi.org/10.1126/science.134.3484.1008)
Schubert, N., Nichols, H., Winternitz, J. (2021). How can the MHC mediate social odor via the microbiota community? A deep dive into mechanisms. Behavioral Ecology, 32, 359–373. [https://doi.org/10.1093/beheco/arab004](https://doi.org/10.1093/beheco/arab004)
Sepil, I., Lachish, S., Sheldon, B. C. (2013). MHC-linked survival and lifetime reproductive success in a wild population of great tits. Molecular Ecology, 22(2), 384-396. [https://doi.org/10.1111/mec.12123](https://doi.org/10.1111/mec.12123)
Sharma, J. M. & Tizard, I. (1984). Avian cellular immune effector mechanisms ‐ A review. Avian Pathology, 13 (3), 357-376. [https://doi.org/10.1080/03079458408418541](https://doi.org/10.1080/03079458408418541)
Sin, S. Y. W., Cloutier, A., Nevitt, G., Edwards, S. V. (2022). Olfactory receptor subgenome and expression in a highly olfactory procellariiform seabird. Genetics, 220 (2), iyab210. [https://doi.org/10.1093/genetics/iyab210](https://doi.org/10.1093/genetics/iyab210)
Slade, J. W. G., Watson, M. J., Kelly, T. R., Gloor, G. B., Bernards, M. A., MacDougall-Shackleton, E. A. (2016). Chemical composition of preen wax reflects major histocompatibility complex similarity in songbirds. Proceedings of the Royal Society B: Biological Sciences, 283 (1842), 20161966. [https://doi.org/10.1098/rspb.2016.1966](https://doi.org/10.1098/rspb.2016.1966)
Sommer, S. (2005). The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front Zool., 2, 16. [https://doi.org/10.1186/1742-9994-2-16](https://doi.org/10.1186/1742-9994-2-16)
Spottiswoode, C. & Møller, A. P. (2004). Extrapair paternity, migration, and breeding synchrony in birds. Behavioral Ecology, 15(1), 41-57. [https://doi.org/10.1093/beheco/arg100](https://doi.org/10.1093/beheco/arg100)
Stapleton, M. K., Kleven, O., Lifjeld, J. T., Robertson, R. J. (2007). Female tree swallows (Tachycineta bicolor) increase offspring heterozygosity through extrapair mating. Behavioral Ecology and Sociobiology, 61(11), 1725-1733. [https://doi.org/10.1007/s00265-007-0404-4](https://doi.org/10.1007/s00265-007-0404-4)
Strandh, M., Westerdahl, H., Pontarp, M., Canbäck, B., Dubois, M.-P., Miquel, C., Taberlet, P., Bonadonna, F. (2012). Major histocompatibility complex class II compatibility, but not class I, predicts mate choice in a bird with highly developed olfaction. Proceedings of the Royal Society B: Biological Sciences, 279(1746), 4457-4463. [https://doi.org/10.1098/rspb.2012.1562](https://doi.org/10.1098/rspb.2012.1562)
Verhulst, S., Dieleman, S. J., Parmentier, H. K. (1999). A tradeoff between immunocompetence and sexual ornamentation in domestic fowl. Proceedings of the National Academy of Sciences, 96(8), 4478-4481. [https://doi.org/10.1073/pnas.96.8.4478](https://doi.org/10.1073/pnas.96.8.4478)
Von Schantz, T., Wittzell, H., Bose, N., Grahn, M., Persson, K. (1996). MHC genotype and male ornamentation: genetic evidence for the Hamilton-Zuk model. Proceedings of the Royal Society of London. Series B: Biological Sciences, 263(1368), 265-271. https://doi.org/10.1098/rspb.1996.0041
Von Schantz, T., Wittzell, H., Göransson, G., Grahn, M. (1997). Mate choice, male condition-dependent ornamentation and MHC in the pheasant. Hereditas, 127 (1-2), 133-140. https://doi.org/10.1111/j.1601-5223.1997.t01-1-00133.x
Wedekind, C. & Folstad, I. (1994). Adaptive or Nonadaptive Immunosuppression by Sex Hormones? The American Naturalist, 143 (5), 936-938. http://www.jstor.org/stable/2462885
Wedekind, C. & Penn, D. (2000). MHC genes, body odours, and odour preferences. Nephrology Dialysis and Transplantation, 15(9), 1269-1271. https://doi.org/10.1093/ndt/15.9.1269.
Westerdahl, H. (2007). Passerine MHC: genetic variation and disease resistance in the wild. Journal of Ornithology, 148(2), 469-477. https://doi.org/10.1007/s10336-007-0230-5
Whittaker, D., Rosvall, K., Slowinski, S., Soini, H., Novotny, M., Ketterson, E. (2018). Songbird chemical signals reflect uropygial gland androgen sensitivity and predict aggression: implications for the role of the periphery in chemosignaling. Journal of Comparative Physiology A, 204. https://doi.org/10.1007/s00359-017-1221-5
Whittaker, D. J. (2022). The secret perfume of the birds: uncovering the science of avian scent. Johns Hopkins University Press.
Whittaker, D. J., Dapper, A. L., Peterson, M. P., Atwell, J. W., Ketterson, E. D. (2012). Maintenance of MHC Class IIB diversity in a recently established songbird population. Journal of avian biology, 43 (2), 109-118. https://doi.org/10.1111/j.1600-048X.2012.05504.x.
Whittaker, D. J. & Hagelin, J. C. (2021). Female-Based Patterns and Social Function in Avian Chemical Communication. Journal of Chemical Ecology, 47 (1), 43-62. https://doi.org/10.1007/s10886-020-01230-1
Whittaker, D. J., Slowinski, S. P., Greenberg, J. M., Alian, O., Winters, A. D., Ahmad, M. M., Burrell, M.J.E., Soin, H.A., Novotny M.V., Ketterson, E.D., Theis, K. R. (2019). Experimental evidence that symbiotic bacteria produce chemical cues in a songbird. Journal of Experimental Biology, 222 (20), jeb202978. https://doi.org/10.1242/jeb.202978
Whittaker, D. J., Soini, H. A., Gerlach, N. M., Posto, A. L., Novotny, M. V., Ketterson, E. D. (2011). Role of testosterone in stimulating seasonal changes in a potential avian chemosignal. Journal of Chemical Ecology, 37, 1349-1357. https://doi.org/10.1007/s10886-011-0050-1
Whittingham, L. A., Freeman-Gallant, C. R., Taff, C. C., Dunn, P. O. (2015). Different ornaments signal male health and MHC variation in two populations of a warbler. Molecular Ecology, 24(7), 1584-1595. https://doi.org/10.1111/mec.13130.
Yamazaki, K., Beauchamp, G. K., Curran, M., Bard, J., Boyse, E. A. (2000). Parent-progeny recognition as a function of MHC odortype identity. Proceedings of the National Academy of Sciences U.S.A., 97(19), 10500-10502. https://doi.org/10.1073/pnas.180320997
Yu, C. Y., Yang, Z., Blanchong, C. A., Miller, W. (2000). The human and mouse MHC class III region: a parade of 21 genes at the centromeric segment. Immunology Today, 21(7), 320-327. https://doi.org/10.1016/s0167-5699(00)01664-9.
Ziegler, A., Kentenich, H., Uchanska-Ziegler, B. (2005). Female choice and the MHC. Trends in Immunology, 26 (9), 496-502. https://doi.org/10.1016/j.it.2005.07.003.
Zinkernagel, R. M. & Doherty, P. C. (1997). The discovery of MHC restriction. Immunology Today, 18 (1), 14-17. https://doi.org/10.1016/s0167-5699(97)80008-4

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Derechos de autor 2024 Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales