Resumen
El dióxido de carbono es una molécula que ha estado presente en la Tierra desde su formación hasta nuestros días y participa en los diferentes ciclos de transformación del carbono. El más importante es el asociado a la conversión de carbono inorgánico a carbono orgánico a través del proceso de fotosíntesis catalizado por la enzima rubisco, el cual permitió el origen de la vida en la Tierra y el almacenamiento de energía solar durante millones de años en lo que hoy llamamos combustibles fósiles. En esta revisión se presenta un breve recuento de los cambios en la concentración atmosférica del CO2 desde su formación hasta nuestros días. Además, se discute la formación y el estudio de las estructuras químicamente complejas que caracterizan algunos de los combustibles fósiles, así como los mecanismos químicos por los cuales se recupera la energía almacenada y se vuelve a generar CO2 al usar dichos combustibles. Se analizan asimismo las implicaciones del uso acelerado de estos recursos en los últimos 150 años, lo que ha llevado el sistema “tierra” a un aumento crítico de la temperatura, con la consecuente necesidad de acciones urgentes para que el incremento sea inferior a 1,5 oC con referencia a la época preindustrial, pues, de no hacerlo, ello tendría consecuencias catastróficas para la vida en la Tierra.
Referencias
Babacan, O., De Causmaecker, S., Gambhir, A., Fajardy, M., Rutherford, A. W., Fantuzzi, A., Nelson, J. (2020). Assessing the feasibility of carbon dioxide mitigation options in terms of energy usage. Nature Energy. 5 (9): 720-728. Doi:10.1038/s41560-020-0646-1
Bassham, J. A., Benson, A. A., Kay, L. D., Harris, A. Z., Wilson, A. T., Calvin, M. (1954). The Path of Carbon in Photosynthesis. XXI. The Cyclic Regeneration of Carbon Dioxide Acceptor1. Journal of the American Chemical Society. 76 (7): 1760-1770. Doi:10.1021/ja01636a012
Beerling, D. J., Kantzas, E. P., Lomas, M. R., Wade, P., Eufrasio, R. M., Renforth, P., Banwart, S. A. (2020). Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature. 583 (7815): 242-248. Doi:10.1038/s41586-020-2448-9
Berkowitz, N. (1985). The Chemistry of Coal. 7: 173-182. United States: Elsevier.
Berner, R. A. (2000). Isotope Fractionation and Atmospheric Oxygen: Implications for Phanerozoic O2 Evolution. Science. 287 (5458): 1630-1633. Doi:10.1126/science.287.5458.1630
Berner, R. A. (2003). The long-term carbon cycle, fossil fuels and atmospheric composition. Nature. 426 (6964): 323-326. Doi:10.1038/nature02131
Daza, C. E., Gallego, J., Moreno, J. A., Mondragón, F., Moreno, S., Molina, R. (2008). CO2 reforming of methane over Ni/Mg/Al/Ce mixed oxides. Catalysis Today. 133-135: 357-366. Doi:10.1016/j.cattod.2007.12.081
Erb, T. J. & Zarzycki, J. (2018). A short history of RubisCO: the rise and fall (?) of Nature’s predominant CO2 fixing enzyme. Current Opinion in Biotechnology. 49: 100-107. Doi: 10.1016/j.copbio.2017.07.017
Espinal, J. F., Mondragón, F., Truong, T. N. (2009). Thermodynamic evaluation of steam gasification mechanisms of carbonaceous materials. Carbon. 47 (13): 3010-3018. Doi:10.1016/j.carbon.2009.06.048
Feulner, G. (2017). Formation of most of our coal brought Earth close to global glaciation. Proceedings of the National Academy of Sciences. 114 (43): 11333-11337. Doi:10.1073/pnas.1712062114
Fondo Monetario Internacional. (2019). Global Fossil Fuel Subsidies Remain Large: An Update Based on Country-Level Estimates. IMF Working Paper WP/19/89. pp 1-37.
Fuss, S., Canadell, J. G., Peters, G. P., Tavoni, M., Andrew, R. M., Ciais, P., Yamagata, Y. (2014). Betting on negative emissions. Nature Climate Change. 4 (10): 850-853. Doi:10.1038/nclimate2392
Gallego, G. S., Mariėn, J. G., Batiot-Dupeyrat, C., Barrault, J., Mondragón, F. (2009). Influence of Pr and Ce in dry methane reforming catalysts produced from La1−xAxNiO3−δperovskites. Applied Catalysis A: General. 369 (1-2): 97-103. Doi:10.1016/j.apcata.2009.09.004
Gallego, G. S., Mondragón, F., Barrault, J., Tatibouët, J.-M., Batiot-Dupeyrat, C. (2006). CO2 reforming of CH4 over La–Ni based perovskite precursors. Applied Catalysis A: General. 311: 164-171. Doi: 10.1016/j.apcata.2006.06.024
Galvez, M. E., Fischer, W. W., Jaccard, S. L., Eglinton, T. I. (2020). Materials and pathways of the organic carbon cycle through time. Nature Geoscienc. 13 (8): 535-546. Doi:10.1038/s41561-020-0563-8
Garciėa, P., Hall, P. J., Mondragón, F. (1999). The use of differential scanning calorimetry to identify coals susceptible to spontaneous combustion. Thermochimica Acta. 336 (1-2): 41-46. Doi:10.1016/s0040-6031(99)00183-5
Gómez-Fernández, B. J., Garciėa-Ruiz, E., Martiėn-Diėaz, J., Gómez de Santos, P., SantosMoriano, P., Plou, F. J., Alcalde, M. (2018). Directed -in vitro- evolution of Precambrian and extant Rubiscos. Scientific Reports. 8 (1): 1-11. Doi: 10.1038/s41598-018-23869-3
Hartmann, J., West, A. J., Renforth, P., Köhler, P., De La Rocha, C. L., Wolf-Gladrow, D. A., Scheffran, J. (2013). Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Reviews of Geophysics. 51 (2): 113-149. Doi: 10.1002/rog.20004
Hayes, J. M. & Waldbauer, J. R. (2006). The carbon cycle and associated redox processes through time. Philosophical Transactions of the Royal Society B: Biological Sciences. 361 (1470):931-950. Doi: 10.1098/rstb.2006.1840
Höök, M., Zittel, W., Schindler, J., Aleklett, K. (2010). Global coal production outlooks based on a logistic model. Fuel. 89 (11): 3546-3558. Doi: 10.1016/j.fuel.2010.06.013
Hügler, M. & Sievert, S. M. (2011). Beyond the Calvin Cycle: Autotrophic Carbon Fixation in the Ocean. Annual Review of Marine Science. 3 (1): 261-289. Doi: 10.1146/annurevmarine-120709-142712
Intergovernmental Panel on Climate Change - IPCC. (2019). Global Warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the lobal response to the threat of climate change, sustainable development, and efforts to eradicate poverty. https://www.ipcc.ch/sr15/
Johnson, B. W. & Wing, B. A. (2020). Limited Archaean continental emergence reflected in an early Archaean 18O-enriched ocean. Nature Geoscience. 13 (3): 243-248. Doi:10.1038/s41561-020-0538-9
Kannappan, B. & Gready, J. E. (2008). Redefinition of Rubisco Carboxylase Reaction Reveals Origin of Water for Hydration and New Roles for Active-Site Residues. Journal of the American Chemical Society. 130 (45): 15063-15080. Doi:10.1021/ja803464a
Karnauskas, K. B., Miller, S. L., Schapiro, A. C. (2020). Fossil Fuel Combustion Is Driving Indoor CO2 Toward Levels Harmful to Human Cognition. Geohealth. 4 (5): 1-21. Doi: 10.1029/2019gh000237
Kasting, J. F. & Ackerman, T. P. (1987). Response: Earth’s Early Atmosphere. Science. 235 (4787): 415b. Doi: 10.1126/science.235.4787.415b
Kheshgi, H. S. (1995). Sequestering atmospheric carbon dioxide by increasing ocean alkalinity. Energy. 20 (9): 915-922. Doi: 10.1016/0360-5442(95)00035-f
Kono, T., Mehrotra, S., Endo, C., Kizu, N., Matusda, M., Kimura, H., Ashida, H. (2017). A RuBisCO-mediated carbon metabolic pathway in methanogenic archaea. Nature Communications. 8 (1): 1-12. Doi: 10.1038/ncomms14007
López, D., Sanada, Y., Mondragón, F. (1998). Effect of low-temperature oxidation of coal on hydrogen-transfer capability. Fuel. 77 (14): 1623-1628. Doi: 10.1016/s0016-2361(98)00086-6
Lüthi, D., Le Floch, M., Bereiter, B., Blunier, T., Barnola, J.-M., Siegenthaler, U., Stocker, T. F. (2008). High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature. 453 (7193): 379-382. Doi: 10.1038/nature06949
Mohr, S. H., Wang, J., Ellem, G., Ward, J., Giurco, D. (2015). Projection of world fossil fuels by country. Fuel. 141: 120-135. Doi: 10.1016/j.fuel.2014.10.030
Molina, A. & Mondragón, F. (1998). Reactivity of coal gasification with steam and CO2. Fuel. 77 (15): 1831-1839. Doi: 10.1016/s0016-2361(98)00123-9
Mondragon, F., Kamoshita, R., Katoh, T., Itoh, H., Ouchi, K. (1984). Coal liquefaction by the hydrogen produced from methanol. Fuel. 63(5): 579-585. Doi: 10.1016/0016-2361(84)90149-2
Mondragón, F., Makabe, M., Itoh, H., Ouchi, K. (1982). Coal liquefaction by the hydrogen produced from methanol. Fuel. 61 (4): 392-393. Doi: 10.1016/0016-2361(82)90058-8
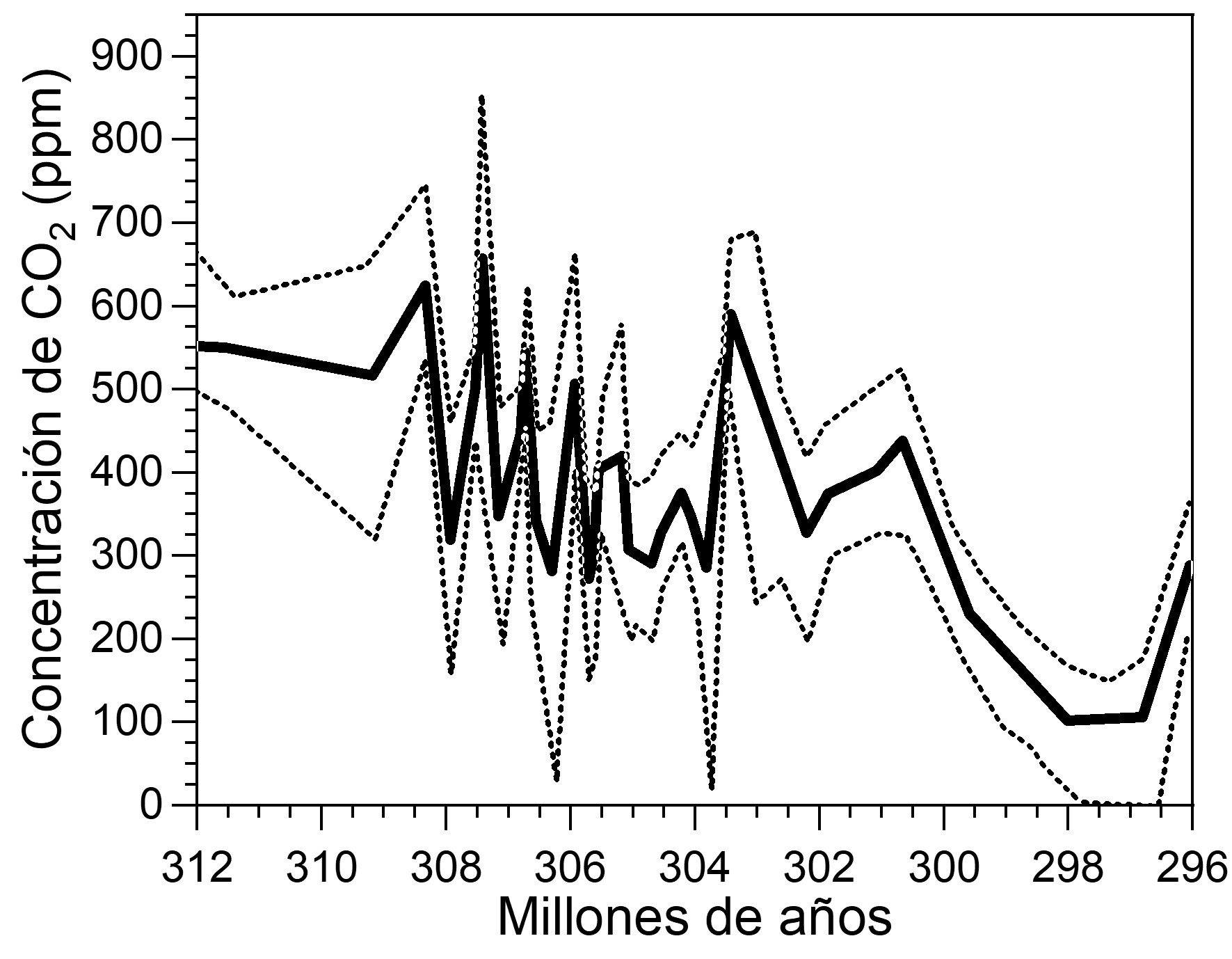
Montañez, I. P., McElwain, J. C., Poulsen, C. J., White, J. D., DiMichele, William A., Wilson, J. P., Hren, M. T. (2016). Climate, pCO2 and terrestrial carbon cycle linkages during late Palaeozoic glacial–interglacial cycles. Nature Geoscience. 9 (11): 824-828. Doi: 10.1038/ngeo2822
Montoya, A., Mondragón, F., Truong, T. N. (2002). First-Principles Kinetics of CO Desorption from Oxygen Species on Carbonaceous Surface. The Journal of Physical Chemistry A. 106 (16): 4236-4239. Doi: 10.1021/jp0144294
Montoya, A., Truong, T.-T. T., Mondragón, F., Truong, T. N. (2001). CO Desorption from Oxygen Species on Carbonaceous Surface: 1. Effects of the Local Structure of the Active Site and the Surface Coverage. The Journal of Physical Chemistry A. 105 (27): 6757-6764. Doi: 10.1021/jp010572l
National Oceanic and Atmospheric Administration - NOAA. (2020). Global Monitoring Laboratory - About Mauna Loa Observatory. Retrieved from https://www.esrl.noaa.gov/gmd/obop/mlo/aboutus/aboutus.html
Orrego, J. F., Zapata, F., Truong, T. N., Mondragón, F. (2009). Heterogeneous CO2Evolution from Oxidation of Aromatic Carbon-Based Materials. The Journal of Physical Chemistry A. 113 (29): 8415-8420. Doi: 10.1021/jp903362g
Parry, M. A. J., Madgwick, P. J., Carvalho, J. F. C., Andralojc, P. J. (2006). Paper Presented At International Workshop On Increasing Wheat Yield Potential, Cimmyt, Obregón, México, 20–24 March 2006. Prospects for increasing photosynthesis by overcoming the limitations of Rubisco. The Journal of Agricultural Science. 145 (1): 31-43. Doi: 10.1017/s0021859606006666
Pérez, S., Mondragón, F., Moreno, A. (2019). Iron ore as precursor for preparation of highly active χ-Fe5C2 core-shell catalyst for Fischer-Tropsch synthesis. Applied Catalysis A: General. 587: 1-8. Doi:10.1016/j.apcata.2019.117264
Quayle, J. R., Fuller, R. C., Benson, A. A., Calvin, M. (1954). Enzymatic Carboxylation of Ribulose Diphosphate1. Journal of the American Chemical Society. 76 (13): 3610-3611. Doi: 10.1021/ja01642a089
Radovic, L. R. (2009). Active Sites in Graphene and the Mechanism of CO2 Formation in Carbon Oxidation. Journal of the American Chemical Society. 131 (47): 17166-17175. Doi: 10.1021/ja904731q
Rasool, S. I. & McGovern, W. E. (1966). Primitive Atmosphere of the Earth. Nature. 212 (5067): 1225-1226. Doi: 10.1038/2121225a0
Renforth, P. & Henderson, G. (2017). Assessing ocean alkalinity for carbon sequestration. Reviews of Geophysics. 55 (3): 636-674. Doi: 10.1002/2016rg000533
Sagan, C. & Mullen, G. (1972). Earth and Mars: Evolution of Atmospheres and Surface Temperatures. Science. 177 (4043): 52-56. Doi: 10.1126/science.177.4043.52
Salamanca, M., Mondragón, F., Agudelo, J. R., Santamariėa, A. (2012). Influence of palm oil biodiesel on the chemical and morphological characteristics of particulate matter emitted by a diesel engine. Atmospheric Environment. 62: 220-227. Doi: 10.1016/j.atmosenv.2012.08.031
Sánchez, A. & Mondragón, F. (2007). Role of the Epoxy Group in the Heterogeneous CO2 Evolution in Carbon Oxidation Reactions. The Journal of Physical Chemistry C. 111 (2): 612-617. Doi: 10.1021/jp065701i
Santamariėa, A., Yang, N., Eddings, E., Mondragón, F. (2010). Chemical and morphological characterization of soot and soot precursors generated in an inverse diffusion flame with aromatic and aliphatic fuels. Combustion and Flame. 157 (1): 33-42. Doi: 10.1016/j.combustflame.2009.09.016
Shih, P. M., Occhialini, A., Cameron, J. C., Andralojc, P. J., Parry, M. A. J., Kerfeld, C. A. (2016). Biochemical characterization of predicted Precambrian RuBisCO. Nature Communications. 7 (1): 1-11. Doi: 10.1038/ncomms10382
Shinn, J. H. (1984). From coal to single-stage and two-stage products: A reactive model of coal structure. Fuel. 63 (9): 1187-1196. Doi: 10.1016/0016-2361(84)90422-8
Suescún-Gómez, D. (1978). Coal deposits in Colombia. In Coal Resources of the Americas Selected Papers. ISBN: 9780813721798, Geological Society of America. Boulder, Colorado (USA). (pp. 49-56).
Tang, H. & Chen, Y. (2013). Global glaciations and atmospheric change at ca. 2.3 Ga. Geoscience Frontiers. 4 (5): 583-596. Doi: 10.1016/j.gsf.2013.02.003
Trigo-Rodriėguez, J. M., Raulin, F., Muller, C., Nixon, C. (2013). The early evolution of the atmospheres of terrestrial planets. New York: Springer Science+Business Media. pp 2-3.
United Nations Framework Convention on Climate Change - UNFCCC. (2015). Paris Agreement. (C.N.464.2017.TREATIES-XXVII.7.d). United Nations Framework Convention on Climate Change. https://treaties.un.org/doc/Publication/CN/2017/CN.464.2017-Eng.pdf
van Krevelen, D. W. (1993). Coal: Typology - Chemistry - Physics - Constitution (3 ed.): Elsevier. Amsterdam ; New York. pp 533 - 701.
Vermeersen, B. L. A., Slangen, A. B. A., Gerkema, T., Baart, F., Cohen, K. M., Dangendorf, S., van der Wegen, M. (2018). Sea-level change in the Dutch Wadden Sea. Netherlands Journal of Geosciences. 97 (3): 79-127. Doi: 10.1017/njg.2018.7

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Derechos de autor 2021 Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales