Resumen
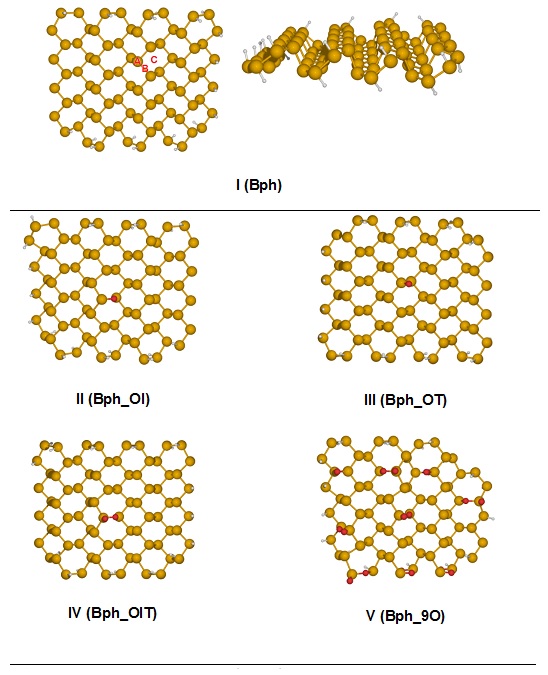
Se hizo un estudio sistemático utilizando la teoría de los funcionales de la densidad (DFT) para lograr una mejor comprensión del papel de la concentración de oxígeno en el fosforeno prístino durante la adsorción de Cu2+ en sistemas acuosos. La caracterización electrónica del fosforeno y del fosforeno oxidado se calculó a partir de la brecha de energía y la dureza química. Los resultados permitieron concluir que los sistemas oxidados presentaron una brecha de energía y una dureza menores que las del sistema prístino. Además, a medida que aumentó la concentración de oxígeno, estos valores decrecieron. La interacción del Cu2+ con las diferentes superficies se caracterizó utilizando cargas atómicas, el índice de enlace y espectroscopia fotoelectrónica de rayos-X (XPS). Los valores de la energía de adsorción indicaron que cuando el fosforeno está oxidado, la interacción con el Cu2+ fue más fuerte comparada con la de la superficie prístina. Asimismo, el aumento en la concentración de oxígeno mejoró las capacidades del fosforeno como adsorbente, lo cual se relaciona con la facilidad que tiene este sistema para la transferencia hacia el Cu2+ dados los reducidos valores de la brecha de energía y la dureza química. Nuestros resultados contribuyen a una mejor comprensión del efecto de la concentración de oxígeno en la superficie de fosforeno en la adsorción de Cu2+, lo que respalda la idea de que este tipo de materiales bidimensionales (2D) tiene uso potencial en la remoción de metales pesados de las aguas residuales.
Referencias
Ajith, M.P., Aswathi, M., Priyadarshini, E., Rajamani, P. (2021). Recent innovations of nanotechnology in water treatment: A comprehensive review. Bioresource Technology, 342, 126000. https://doi.org/10.1016/j.biortech.2021.126000
Becke, A.D. (1993). Density-functional thermochemistry. III. The role of exact exchange. The Journal of Chemical Physics, 98, 5648. https://doi.org/10.1063/1.464913
Chen, O.P., Lin, Y. J., Cao, W. Z., Chang, C. T. (2017). Arsenic removal with phosphorene and adsorption in solution. Materials Letters, 190, 280-282. https://doi.org/10.1016/j.matlet.2017.01.030
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., . . . Fox, D.J. (2009). Gaussian 09 Revision E.01. Gaussian, Inc., Wallingford CT.
Gómez-Pérez, J.F., Correa, J.D., Bartus Pravda, C., Kónya, Z., Kukovecz, Á. (2020). Danglingto-Interstitial Oxygen Transition and Its Modifications of the Electronic Structure in Few-Layer Phosphorene. Journal of Physical Chemistry C, 124(44), 24066-24072. https://doi.org/10.1021/acs.jpcc.0c06542
Hamid, Y., Liu, L., Usman, M., Naidu, R., Haris, M., Lin, Q., Ulhassan, Z., Hussain M.I., Yang, X. (2022). Functionalized biochars: Synthesis, characterization, and applications for removing trace elements from water. Journal of Hazardous Materials, 437, 129337. https://doi.org/10.1016/j.jhazmat.2022.129337
Hoangh, A.T., Nizetic, S., Cheng, C.K., Luque, R., Thomas, S., Banh, T.L., Pham,V.V., Nguyen, X.P. (2022). Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: A comprehensive review. Chemosphere, 287, 131959. https://doi.org/10.1016/j.chemosphere.2021.131959
Huang, Y. H., Hsueh, C. L., Cheng, H. P., Su, L.C., Chen, C. Y. (2007). Thermodynamics and kinetics of adsorption of Cu(II) onto waste iron oxide. Journal of Hazardous Materials, 144, 406-411. https://doi.org/10.1016/j.jhazmat.2006.10.061
Keith, T.A., Frisch, M.J. (1994). Inclusion of Explicit Solvent Molecules in a Self-Consistent-Reaction Field Model of Solvation. En D. A. Smith, Modeling the Hydrogen Bond (pp 22-35). American Chemical Society. https://doi.org/10.1021/bk-1994-0569.ch003 Kharwar, S., Singh, S. (2021). First-principles investigation of zigzag graphene nanoribbons based nanosensor for heavy metal detector. Materials Today: Proceedings, 47, 2227-2231. https://doi.org/10.1016/j.matpr.2021.04.183
Koopmans, T. (1934). Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica, 1, 104-113. https://doi.org/10.1016/S0031-8914(34)90011-2
Liaquat, H., Imran, M., Latif, S., Hussain, N., Bilal, M. (2022). Multifunctional nanomaterials and nanocomposites for sensing and monitoring of environmentally hazardous heavy metal contaminants. Environmental Research, 214, 113795. https://doi.org/10.1016/j.envres.2022.113795
Mason, L.H., Harp, J. P., Han, D.Y. (2014). Pb Neurotoxicity: Neuropsychological Effects of Lead Toxicity. BioMed Research International, 214, Article ID 840547, 8 pages. https://doi.org/10.1155/2014/840547
Menazea, A.A., Ezzat, H.A., Omara, W., Basyouni, O.H., Ibrahim, S. A., Mohamed, A.A., Tawfik, W., Ibrahim, M.A. (2020). hitosan/graphene oxide composite as an effective removal of Ni, Cu, As, Cd and Pb from wastewater. Computational and Theoretical Chemistry 1189, 112980. https://doi.org/10.1016/j.comptc.2020.112980
Pan, J., Gao, B., Guo, K., Gao, Y., Xu, X., Yue, Q. (2022). Insights into selective adsorption mechanism of copper and zinc ions onto biogas residue-based adsorbent: Theoretical calculation and electronegativity difference. Science of the Total Environment, 805, 150413. https://doi.org/10.1016/j.scitotenv.2021.150413
Parr, R.G., Pearson, R.G. (1983). Absolute hardness: companion parameter to absolute electronegativity. Journal of the American Chemical Society, 105, 7512-7516.https://doi.org/10.1021/ja00364a005
Pearson, R.G. (2005). Chemical hardness and density functional theory. Journal of Chemical Sciences volume, 117, 369-377. https://doi.org/10.1007/BF02708340
Perdew, J.P., Burke, K., Wang, Y. (1996). Generalized gradient approximation for the exchangecorrelation hole of a many-electron system. Physycs Review B, 54(23), 16533-16539. https://doi.org/10.1103/PhysRevB.54.16533
Reed, A.E., Curtiss, L.A., Weinhold, F. (1988). Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chemical Reviews, 8, 899-926. https://doi.org/10.1021/cr00088a005
Srivastava, M., Srivastava, A. (2021). DFT analysis of nitrogen and Boron doped Graphene sheet as lead detector. Materials Science and Engineering B, 269, 115165. https://doi.org/10.1016/j.mseb.2021.115165
Ugwu, E.I., Othmani, A., Nnaji, C.C. (2022). A review on zeolites as cost-effective adsorbents for removal of heavy metals from aqueous environment. International Journal of Environmental Science and Technology, 19, 8061-8084. https://doi.org/10.1007/s13762-021-03560-3
Ullah, N., Mansha, M., Khan, I., Qurashi, A. (2018). Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. Trends in Analytical Chemistry, 100, 155-166. https://doi.org/10.1016/j.trac.2018.01.002
Uogintė, I., Lujanienė, G., Mažeika, K. (2019). Study of Cu (II), Co (II), Ni (II) and Pb (II) removal from aqueous solutions using magnetic Prussian blue nano-sorbent. Journal of Hazardous Materials. Journal of Hazardous Materials, 269, 226-235. https://doi.org/10.1016/j.jhazmat.2019.02.039
Wang, X., Kong, L., Zhou, S., Ma, C., Lin, W., Sun, X., Kirsanov, D., Legin, A., Wan, H., Wang, P. (2022). Development of QDs- based nanosensors for heavy metal detection: A review on transducer principles and in- situ detection. Talanta, 239, 122903. https://doi.org/10.1016/j.talanta.2021.122903
Weigend, F., Ahlrichs, R. (2005). Balanced basis sets of split valences, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Physical Chemistry Chemical Physics, 7, 3297-3305. https://doi.org/10.1039/B508541A
Wijaya, A.R., Ouchi, A. K., Tanaka, K., Cohen, M.D., Sirirattanachai, S., Shinjo, R., Ohde, S. (2013). Evaluation of heavy metal contents and Pb isotopic compositions in the Chao Phraya River sediments: Implication for anthropogenic inputs from urbanized areas, Bangkok. Journal of Geochemical Exploration, 126-127, 45-54. https://doi.org/10.1016/j.gexplo.2012.12.009
Zhao, Y., Truhlar, D. (2008). Density Functionals with Broad Applicability in Chemistry. Acc.Chem. Res, 41(2), 157-167. https://doi.org/10.1021/ar700111a

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Derechos de autor 2023 Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales